Table of Contents
Robotic Laryngeal Surgery
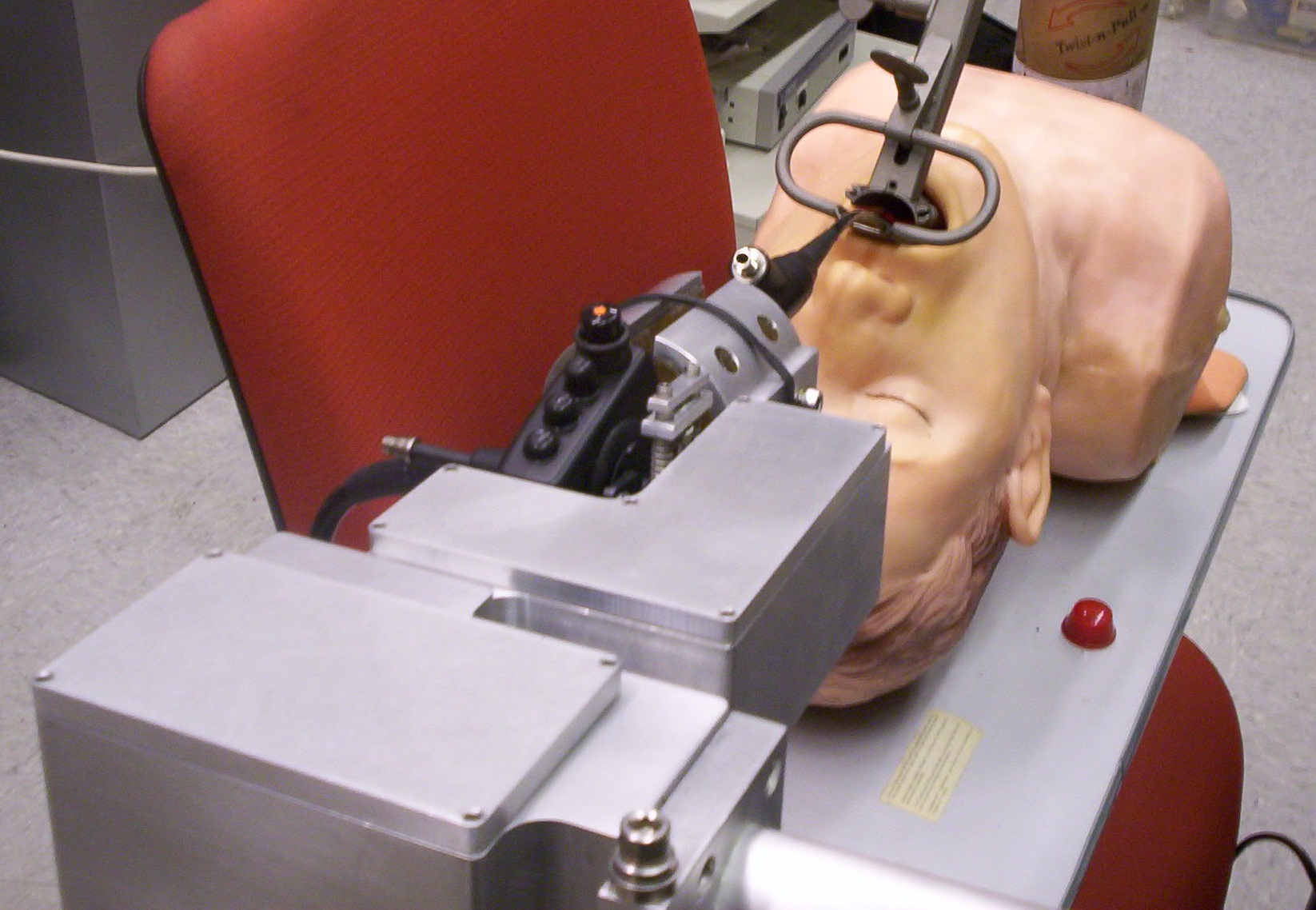 Over 25,000 new cases of throat cancer are reported every year in the US, resulting in approximately 6,000 deaths per year 1). Since the throat area is fraught with complications for many cancer treatment techniques, such as radiation and chemotherapy, but relatively accessible to surgical techniques, a surgical approach is often taken. Two main surgical techniques are used: conventional surgery, and intra-airway surgery. Intra-airway surgery has many advantages over conventional surgery, including faster healing, less chance of infection, less visible scarring, and less risk of complications, but due to the confined space and poor visibility in the airway, such surgeries are often difficult and time consuming to perform. These difficulties can be partially overcome by using a flexible laryngoscope with a working port, but the problem of adequately controlling the scope with millimeter accuracy for sometimes hours at a time remains. This project seeks to solve this problem by using a robot to control the laryngoscope, thus allowing the surgeon to bypass the details of actuating the scope and focus on the higher level goals of the operation.
Over 25,000 new cases of throat cancer are reported every year in the US, resulting in approximately 6,000 deaths per year 1). Since the throat area is fraught with complications for many cancer treatment techniques, such as radiation and chemotherapy, but relatively accessible to surgical techniques, a surgical approach is often taken. Two main surgical techniques are used: conventional surgery, and intra-airway surgery. Intra-airway surgery has many advantages over conventional surgery, including faster healing, less chance of infection, less visible scarring, and less risk of complications, but due to the confined space and poor visibility in the airway, such surgeries are often difficult and time consuming to perform. These difficulties can be partially overcome by using a flexible laryngoscope with a working port, but the problem of adequately controlling the scope with millimeter accuracy for sometimes hours at a time remains. This project seeks to solve this problem by using a robot to control the laryngoscope, thus allowing the surgeon to bypass the details of actuating the scope and focus on the higher level goals of the operation.
Project Goals
The main goal of this project is to create a robotic scope manipulation system for laryngeal surgery. The be clinically useful, such a system would need to:
- have equal or superior precision to manual scope manipulation
- pass all clinical regulatory requirements
- be rugged enough to withstand typical operating room conditions
- hold the scope steady even when the surgeon releases the system to attend to other tasks
- be easy and simple for users with little or no technical background to set up and use
- introduce no unnecessary risks into the procedure
Approach
Our approach includes a custom built robot to manipulate the endoscope and a software system to allow the surgeon to easily manipulate the scope using only one hand. The RoboELF is a cheap and simple solution to partially automate a simple surgical task.
Status and Results to Date
We created an initial proof of concept prototype using the LARS robot over the summer of 2009. Once this prototype was tested and approved, we began work on a ruggedized clinical prototype in fall of 2009. The first iteration of the ruggedized prototype was completed in spring 2010. After further testing and evaluation, we began the second iteration of the ruggedized prototype, which includes several enhancements and modifications.
Video of First Iteration RoboELF
We submitted an inquiry to the FDA in 2011 to determine the risk level of the RoboELF for human clinical trials. Based on their response, we implemented further design updates to the physical robot and the software system.
Physical Changes:
- Draping Procedure
- Redesigned Scope Holder Mechanism
- New Joystick control mechanism
- Housing for joysticks and EStop switch
- Breakup of large parts for easier assembly and storage
Software Changes:
- More robust watchdog timer
- More robust error checking/handling
- Consistency checking of Encoders/Potentiometers on robot joints
- Major code refactoring and cleanup
We also compiled further documentation including an FMEA, testing plan and user manual. These changes and documents were completed and reviewed in winter 2012. The FDA has classified the system as a low risk device, so we are now awaiting IRB approval for a human subjects study.
Project Personnel
Students:
- Kevin Olds, BME PhD candidate
Advisors:
- Jeremy Richmon MD
- Russell Taylor
Funding
- Project is supported by the JHMI Department of Otolaryngology
Other Links
Publications
- Olds, K., Hillel, A. T., Cha, E., Curry, M., Akst, L. M., Taylor, R. H. and Richmon, J. D. (2011), “Robotic endolaryngeal flexible (Robo-ELF) scope: A preclinical feasibility study.” The Laryngoscope, 121: 2371–2374.
- Olds, Kevin; Hillel, Alexander; Kriss, Jonathan; Nair, Archana; Kim, Hongho; Cha, Elizabeth; Curry, Martin; Akst, Lee; Yung, Rex; Richmon, Jeremy; Taylor, Russell. “A robotic assistant for trans-oral surgery: the robotic endo-laryngeal flexible (Robo-ELF) scope.” Journal of Robotic Surgery vol. 6 issue 1 March 2012. p. 13 - 18

