Contact Us
CiiS Lab
Johns Hopkins University
112 Hackerman Hall
3400 N. Charles Street
Baltimore, MD 21218
Directions
Lab Director
Russell Taylor
127 Hackerman Hall
rht@jhu.edu
Last updated: 18 April 2017
A method to dynamically position x-ray beam filters for low dose CT acquisitions is needed in clinical scenarios in which manual centering of the patient within the bore is impractical. Traditional bowtie filters allow the reduction of dose received by the patient without loss of image quality, but these benefits are lost when the physician is forced to remove the filter in the interest of time, which may be common in the emergency room. Dr. J Web Stayman of the AIAI Laboratory has developed a multiple aperture device, a novel beam filter with a simple actuation mechanism that can be integrated into commercial CT scanners. This project’s goal is to develop a system that can automatically determine the patient’s position and dynamically position the x-ray beam, bringing back the benefits of low-dose CT acquisitions to the ER.
The great diagnostic utility of x-ray CT has led to dramatically increased use over the past decade. The associated increase in population radiation dose measurements has garnered significant public attention over the development of dose reduction methods. The number of CT procedures per year has been increasing at an annualized rate >10%, and while CT represents only 15% of radiological exams using ionizing radiation, it accounts for over 50% of the effective dose.
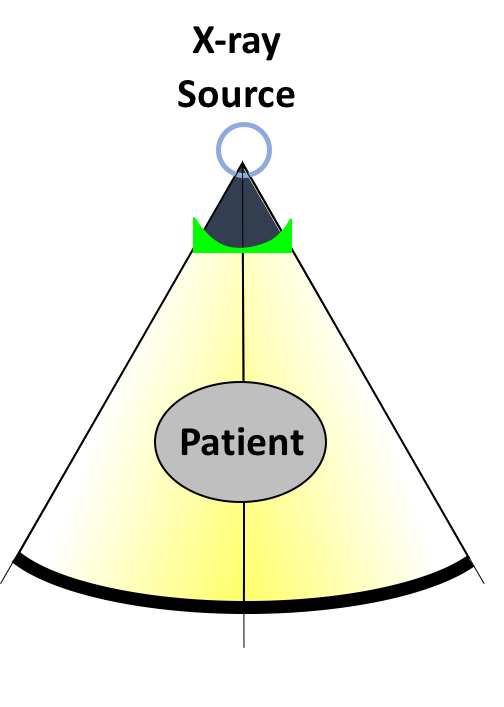 Figure 1. Bowtie filters in CT.
Figure 1. Bowtie filters in CT.
Traditional CT acquisition methods have significant problems leading to increased effective dose. Existing clinical CT scanners are limited in their ability to customize data acquisitions to the patient, as CT studies are usually ordered with a “one-size fits all” mentality. Optimal data acquisition strategies vary from patient to patient and based on the anatomical site or imaging task, but the lack of any ability to create spatially varying x-ray beam fluence profile has significant radiation dose consequences: delivering more radiation than is needed to accomplish a medical diagnosis. Because an axial slice of the patient can be seen to be ellipsoidal in shape, it is easy to see that there is less attenuating tissue at the sides of the patient than in the center. As a result, an x-ray source delivering the same beam intensity in all directions towards the patient will cause the extremities to be overexposed, which has both dose and image quality consequences. A simple solution to promote a uniform beam profile arriving at the detector is the use of a bowtie filter, shown in Figure 1. The bowtie filter is designed simply to attenuate the beam more heavily at the sides of the patient, and can be made from a variety of materials, shown in Figure 2. A custom designed multiple aperture device (MAD), a novel beam filter developed in the AIAI laboratory, is also shown (to be discussed in more detail).
Figure 2. (a) Bowtie filters. (b) Microfabricated tungsten MAD filter.
While bowtie filters are commonly used in CT scanners today, they are incorporated into CT gantries spinning at fast revolutions that do not allow their translation with respect to the patient imaging plane. This creates an issue when the patient is not centered within the bore of the scanner, resulting again in the spatial misplacement of x-ray beam fluence. This scenario can result in the highly undesirable effect of increased dose in certain areas of the body, as well as increased noise in certain areas of the resulting tomograph [4]. Habibzadeh et. al. have shown that miscentering of an average of 3 cm below the center can cause a 25.8% increase in dose and an 8.3% increase in noise, shown in Figure 3 below [6]. Toth et. al. also showed in real clinical data that lateral positioning errors can range from -2.9 cm to 3.3 cm, and elevation errors from - 6.6 to 3.4 cm [4].
Figure 3. a) Miscentered patient. b) Increased noise in lower part of reconstructed image [4]
Clinically, centering patients within the bore is an error-strewn process that sometimes necessitates re-centering and retaking of images. While this by itself can lead to increased radiation dose, a larger need arises in imaging for emergency medicine, where time is of the essence and physicians cannot afford to spend extra time positioning the patient when making a diagnosis. Additionally, it is necessary for physicians to visualize the entire volume of the body with high image quality to make an effective diagnosis in the emergency room. For these reasons, it is common for the bowtie filter to be removed when imaging in the ER, resulting in increased dose and the loss of the benefits of beam filtration. This scenario thus necessitates an automatic method for determining the patient’s position within the bore and dynamic beam filter positioning for low-dose CT acquisitions in emergency medicine applications.
To overcome limitations preventing translation of the bowtie filters within existing clinical CT gantries, the AIAI lab has developed novel dynamic beam modulation hardware called the multiple-aperture device (MAD). The MADs are designed to be capable of dynamically adjusting the beam profile and centering in each projection during gantry based on Moiré patterns created when two MADs are translated with respect to each other. In this project, however, we will be using only a single MAD to evaluate the efficacy of our system to avoid the extra complications associated with having two dynamic MADs.
Our aim is to achieve dynamic x-ray beam positioning for low-dose CT acquisitions and quantitative performance assessment for arbitrary patient positioning in emergency medicine applications.
Design
The general approach for our system is shown below. We will first determine the patient position within the field of view by acquiring low dose “scout” radiograph scans at two views 90° apart. Using this estimate of the patient’s position, the beam filter trajectory over the 360° acquisition can be computed. Then, image acquisition will take place with the beam filter dynamically positioned during the acquisition arc. Images will then be reconstructed with the proper associated data corrections with FDK reconstruction (filtered back-projection) as a baseline. All code will be implemented in MATLAB, with functions for each of our different steps.
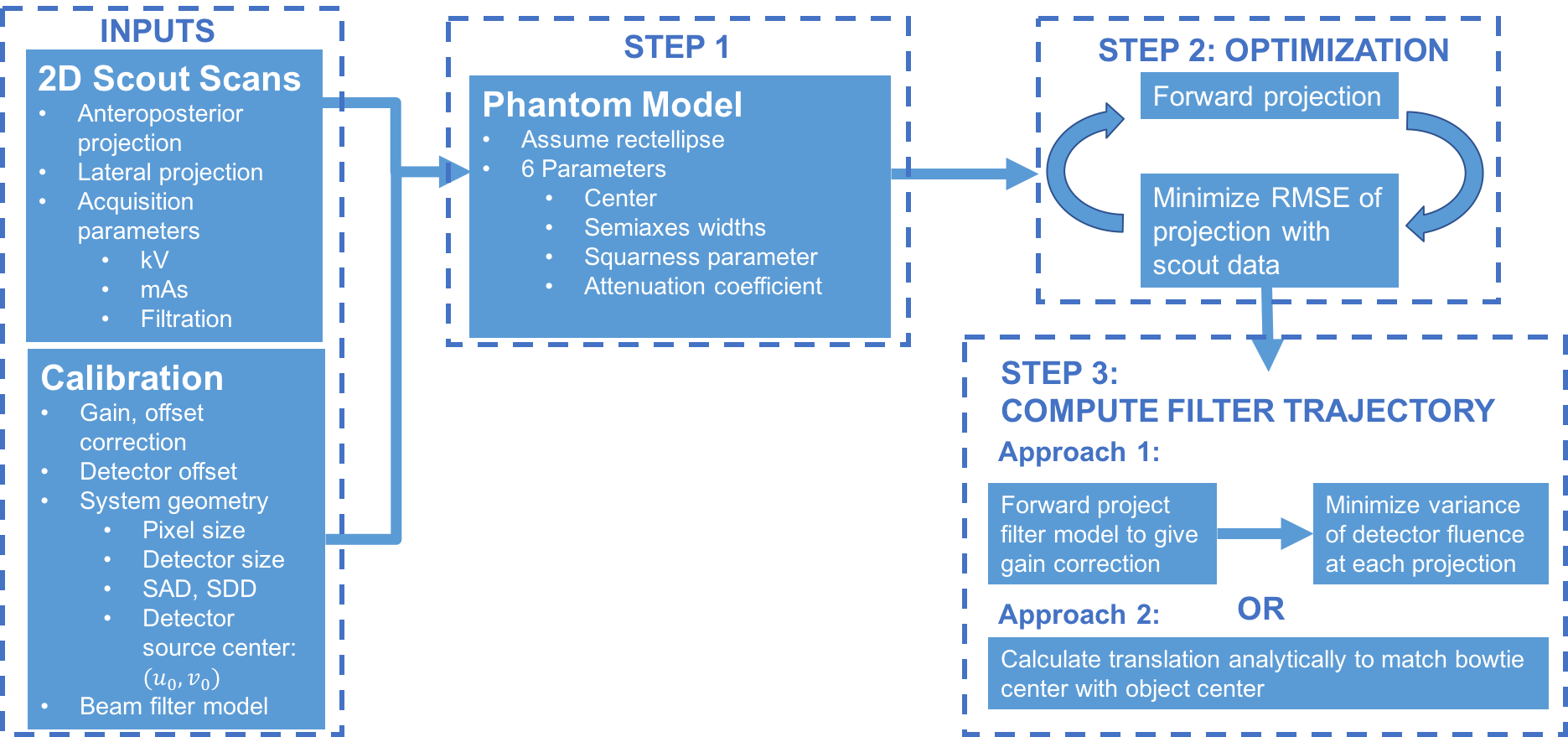 Figure 4. Block diagram of technical approach for dynamic beam positioning.
Figure 4. Block diagram of technical approach for dynamic beam positioning.
Our approach begins with taking two low-dose scout images of the object from antero- posterior (AP) and lateral views. These two scout views are already routinely taken in CT imaging, which means there is no conflict with standard clinical imaging protocol. To compute the position of the object within the FOV from two low-dose scout scans, several assumptions need to be made. First, the object in the axial slice will be assumed to be generally of an ellipsoid shape. Then, the bowtie will be fixed at a certain distance zbowtie from the source (realistic as this is unlikely to be a degree of freedom in a commercial scanner), where the shape and material of the bowtie is known. Using this assumption, the projection data obtained at any view will be a function of the position of the center of the object c0 = ( x0, y0 ) in the axial plane, the width w and height h of the ellipse in the axial plane, as well as the gross attenuation coefficient μ. Using the two views, these 5 parameters can be estimated either using an optimization process by minimizing ||P x - g||2 with respect to x. where P is forward projection x operator and g is the data, or analytically using projection equations of rays emanating from the source. The geometry is shown below in Figure 5.
Once the model of the ellipse has been determined, an optimal beam filter trajectory can be computed given the shape of the bowtie being used and zbowtie. Specifically, at each angular position in the acquisition, an optimization process can be performed on the translational position of the bowtie that will produce the flattest fluence profile at the detector. The translational plane of motion for the bowtie is also shown as a dashed line in Figure 5 above. Once the beam filter trajectory is computed, the acquisition can be performed.
Image reconstructions will be performed using the baseline FDK algorithm, which is based on filtered backprojection of cone beam CT data. A GPU implementation of the algorithm is provided in the CUDA Tools developed by the I-STAR laboratory, and is one of our project dependencies (which we have met). For acquisitions using the bowtie filters, the major difference from traditional acquisitions is that the incident intensity I0 needs to be accurately determined at each projection. Given the beam filter trajectory and the shape of the beam filter, this can be accurately calculated from traditional gain scan data and is independent of the specific phantom or its position within the FOV. We will simply need to perform this preprocessing step before using our projection data with the FDK reconstruction software from CUDA Tools.
However, the reconstruction process is much more complicated with the MADS, due to the high frequency pitch of the gratings. The MADs are designed to have a pitch that is matched to the size of the –x-ray tube focal spot, and are focused and positioned such that the high frequency gratings are blurred out in the fluence profile arriving at the object. However, small positional shifts or blooming effects of the focal spot during the acquisition prevent an ideal blurring of the MAD gratings and result in high frequency artifacts in the projection data. This results in ringing artifacts in the reconstructed images, as shown in Figure 6. These effects will need to be thoroughly studied and modeled, and this artifact correction will be performed in parallel to other work in this project as part of Andrew’s Master’s thesis research in the AIAI laboratory. Pending resolution of the MAD artifact corrections, we will then be able to achieve our maximum deliverable of acquiring phantom data with a single MAD. We do not propose to do acquisitions with the dual MADs because it will not be necessarily to dynamically modulate the fluence field at each projection (i.e., the baseline profile provided by the single MAD will be sufficient to demonstrate our project).
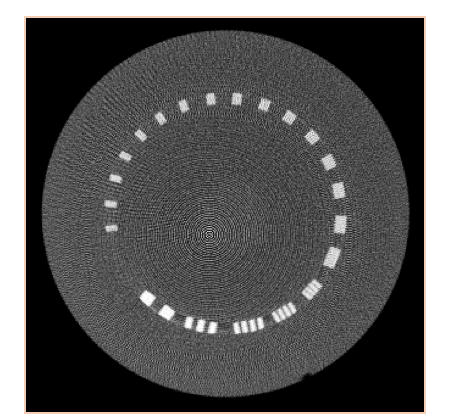 Figure 6. Ringing artifacts in MAD reconstruction
Figure 6. Ringing artifacts in MAD reconstruction
Evaluation
Validation of our system’s efficacy will take place using a series of acquisition experiments on phantoms using both bowtie (expected deliverable) and MAD (maximum deliverable) beam filters. To do this, we will need to properly assess the performance of our imaging system by computing both image quality metrics (including SNR, CNR, spatial resolution and noise) as well as making dose measurements (measured separately in CTDI phantoms) and comparing both to traditional CT acquisition methods in the ER (i.e., without any beam filters). This will involve acquiring phantom images with no filter, a bowtie, and a single MAD at a minimum of 5 different positions corresponding to different off-center shifts of the object. This will then allow us to generate plots of image quality metrics and dose over the different off-center shifts under each of the three acquisition conditions. The cone beam CT (CBCT) test bench setup in the AIAI lab that we will use to do our acquisitions is shown in Figure 7 below.
Dependencies for Simulations and Image Reconstruction
Dependencies for Physical Phantoms
Advising Dependencies
1. Stayman, J. Webster, Mathews, Aswin, Zbijewski, Wojciech, Gang, Grace J., Siewerdsen, Jeffrey H., Kawamoto Satomi, Blevis, Ira, Levinson, Reuven (2016): Fluence-field modulated x-ray CT using multiple aperture devices. In: Kontos, Despina; Flohr, Thomas; Lo, Joseph (Ed.): SPIE Medical Imaging, pp. 97830X,International Society for Optics and Photonics 2016.
2. Mathews, Aswin, Tilley II, Steven, Gang, Grace J., Kawamoto, Satomi, Zbijewski, Wojciech, Siewerdsen, Jeffrey H., Levinson, Reuven, Stayman, J. Webster(2016): Design of dual multiple aperture devices for dynamical fluence field modulated CT. In: 4th International Conference on Image Formation in X-Ray Computed Tomography, pp. 29–32, 2016.
3. Ouadah, Sarah, Stayman, J. Webster, Gang, Grace J., Ehtiati, Tina, Siewerdsen, Jeffrey H. (2016): Self-calibration of cone-beam CT geometry using 3D-2D image registration.. In: Physics in medicine and biology, 61 (7), pp. 2613–32, 2016, ISSN: 1361-6560.
4. Toth, T., Ge, Z. and Daly, M. P. (2007), The influence of patient centering on CT dose and image noise. Med. Phys., 34: 3093–3101. doi:10.1118/1.2748113
5. Brenner DJ, Hall EJ. Computed Tomography — An Increasing Source of Radiation Exposure. 2017:2277-2284. 6. Habibzadeh MA, Ay MR, Kamali AR, Ghadiri H, Zaidi H. The Influence of Patient Miscentering on Patient Dose and Image Noise in Two Commercial CT Scanners. 2010:327-330.
7. Pari V. Pandharipande, Andrew T. Reisner, William D. Binder, Atif Zaheer, Martin L. Gunn, Ken F. Linnau, Chad M. Miller, Laura L. Avery, Maurice S. Herring, Angela C. Tramontano, Emily C. Dowling, Hani H. Abujudeh, Jonathan D. Eisenberg, Elkan F. Halpern, Karen Donelan, and G. Scott Gazelle. CT in the Emergency Department: A Real-Time Study of Changes in Physician Decision Making. Radiology 2016 278:3, 812-821
8. Gies, M., Kalender, W. A., Wolf, H., Suess, C. and Madsen, M. T. (1999), Dose reduction in CT by anatomically adapted tube current modulation. I. Simulation studies. Med. Phys., 26: 2235–2247. doi:10.1118/1.598779
Here give list of other project files (e.g., source code) associated with the project. If these are online give a link to an appropriate external repository or to uploaded media files under this name space.
https://git.lcsr.jhu.edu/amao2/cis2_dynamicbeampositioning/tree/master