Stroke Rehabilitation Hand Device
Summary
Patients that have been affected by stroke improve most in motor skills when treatment is administered within the first couple months after the incident. By introducing a user-friendly, portable, and relatively low-cost device that can record and process small forces from the fingers that are typically characteristic of severe stroke cases, early intervention and treatment of stroke becomes more practical and feasible for a larger demographic of patients.
Students: Jacob Carducci
Mentor(s): Kevin Olds

Background, Specific Aims, and Significance
Arguably one of the more debilitating injuries are lesions that affects the strength and control of the hands and upper limbs, which makes it difficult to perform everyday tasks including grabbing objects and interacting with computers. In the case of computer interface, stroke affected patients may not be able to target with a single finger and slide with sufficient range on a touchscreen. Some patients may not even feel the sense of touch when interacting with a tactile screen, making the act of consistent pressing difficult. Researchers at Johns Hopkins’ own Department of Neurology have recently completed a study into post-stroke recovery of the human hand, which has revealed that both strength and control in the hand generally improves the most within the first three months (or twelve weeks) of recovery. Therefore, it is key to introduce rehabilitation to the patient as early as possible to improve the chances and effectiveness of recovery, which can lead to a better quality of life for the patient.
Goals of this project include:
Getting clinical study approval from the hospital’s clinical engineering team and the IRB, which will allow for studies to quantify the effectiveness of the current and future prototypes
Designing and fabricating an improved version of the device based on study feedback, which should increase the number of fingers that forces are tracked from, increase the accuracy of forces detected from the thumb, and make the device easier to disassemble and clean for stroke-affected individuals living independently
Getting preliminary study feedback for the revised prototype to see if the changes made increase the effectiveness of the rehabilitation device
Deliverables
Minimum: (05/15/2017)
Correspondence and approval documents from clinical engineering and IRB for initial study
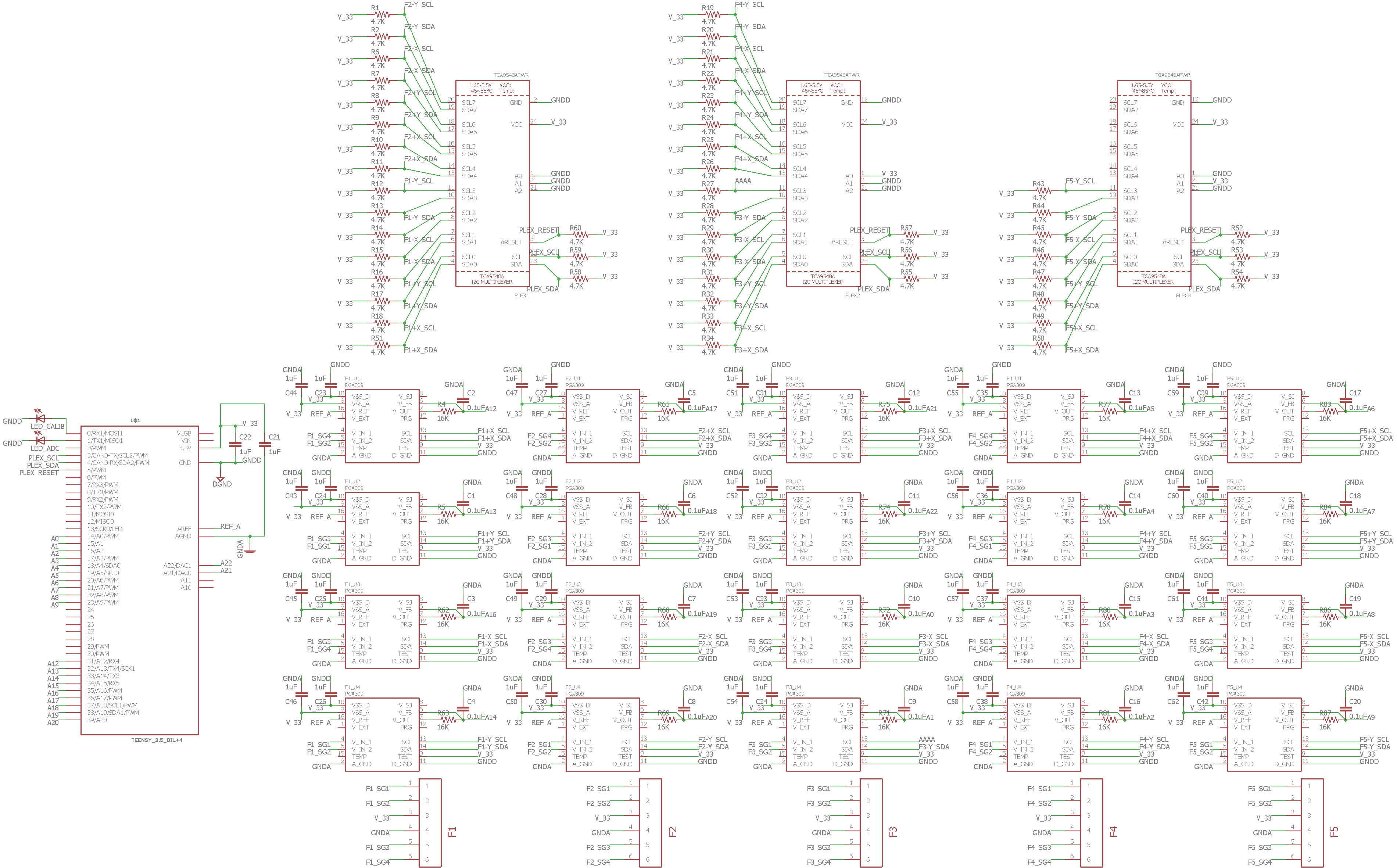
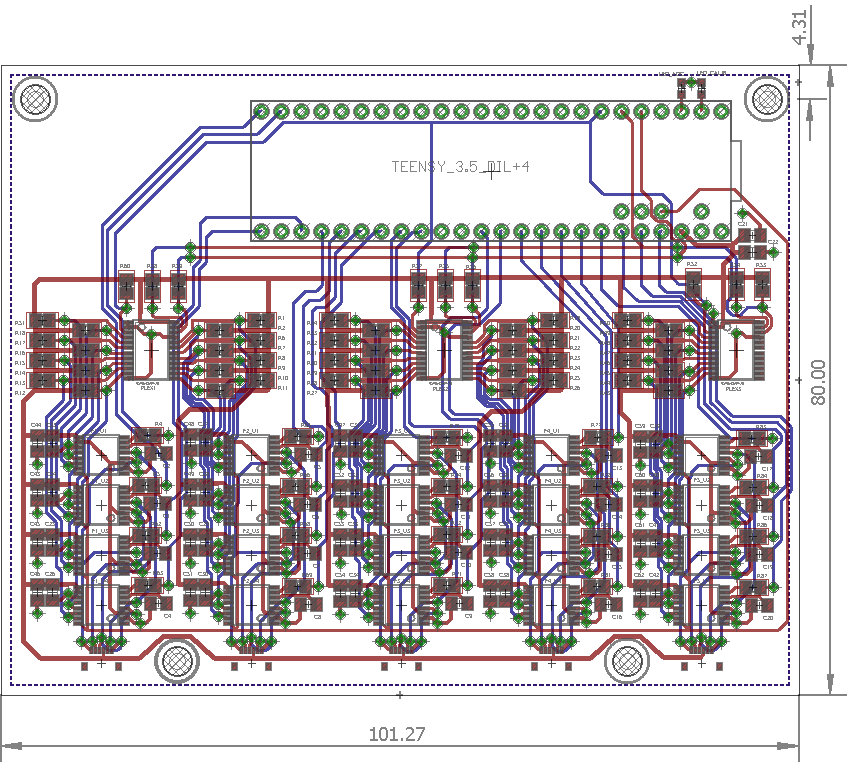
Schematics and diagrams of each subsystem
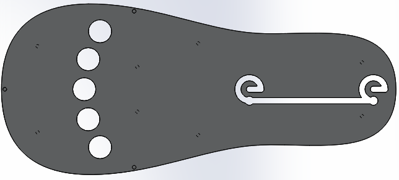
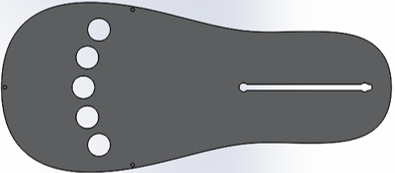
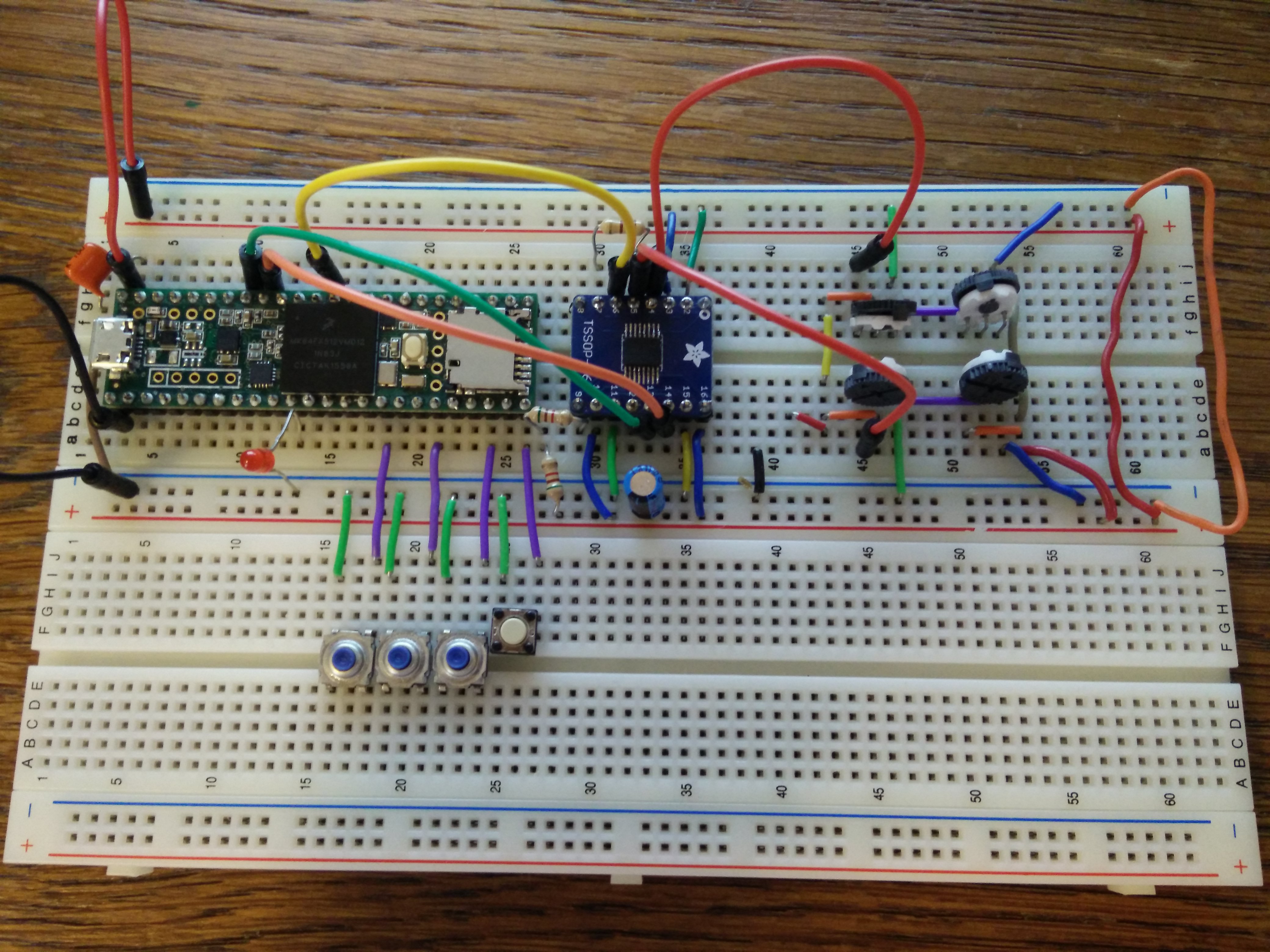
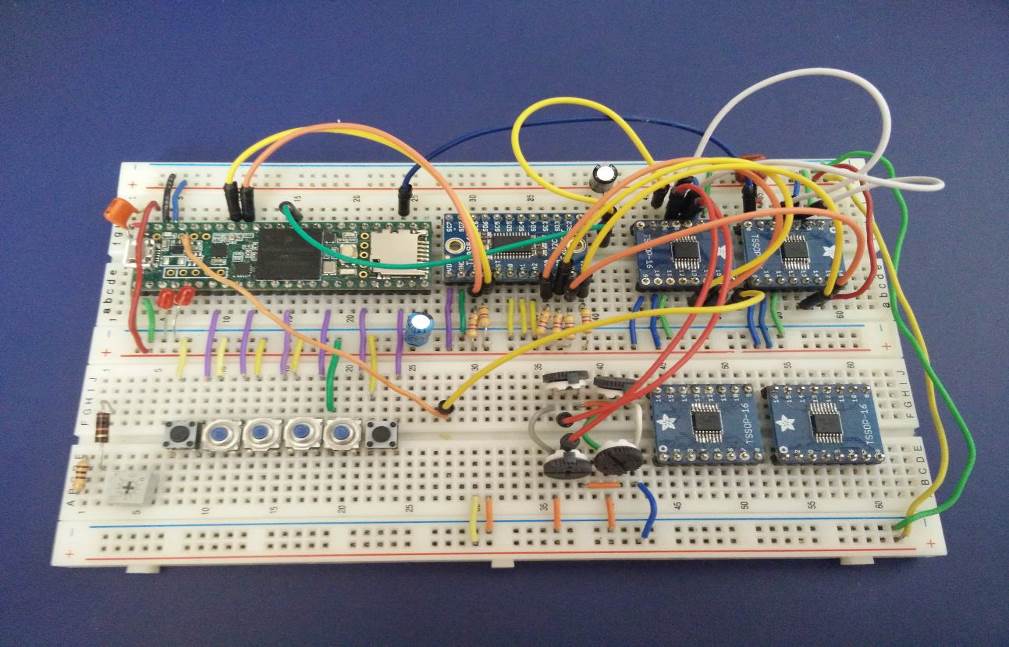



Force test results from initial prototype
Expected: (05/20/2017)
Assembled and functional revised prototype (2 identical copies)
Maximum: (Summer 2017)
Correspondence and approval documents from clinical engineering and IRB for second study
Force test results from revised prototype
Technical Approach
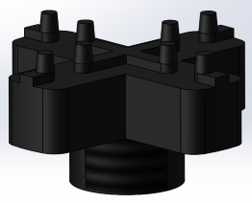
Patients will have their hand of focus fitted into an adjustable wrist brace that locks into the base of the rehab tool. The fingers of the patient will fit into up to five adjustable silicon retention cups. The base of each cup structure will contain a strain-gauge force-sensor array that can detect pressing, lifting, and lateral pressures from the patient at a high sensitivity to account for the potential weakness of the patient. The signal changes from the sensor arrays are sent to a controller board inside the tool and then to a connected computer, which processes the signal fluctuations into useful information that can be used in higher-level applications or games.
Dependencies
UPDATE: All needed parts have been ordered and received, so this dependency has been resolved.
Delays in the approval process for CES and IRB may occur despite the low risk posed to users by the device, which could impact the completion of studies. If delays are frequent and/or are too long, the project can shift to a more technical focus rather than a clinical focus, and deliverables will be adjusted to reflect that.
UPDATE: Due to a transition in clinical study focus to a collaboration with Western University, current progress on IRB and CES approval has been put on hold until further notice. Therefore, the project has indeed shifted to a more technical focus with design being driven by techincal objectives and client needs rather than direct patient feedback.
Even with CES and IRB approval, there can be delays in recruiting patients to participate in the study due to proximity, availability, and meeting certain criteria. This can also impact the completion of studies. If this occurs, many aspects of the design can be tested with healthy subjects, which are much easier to recruit.
UPDATE: Western University will deal with much of the patient selection for its own study, but this dependency may become relevant again if an American counterpart to this study is developed.
Milestones and Status
Milestone name: Clinical engineering team (CES) approval stamp
Planned Date: 03/03/2017
Expected Date: 03/20/2017
Status: “Resolved”. My personal contribution to the CES report is complete, but is no longer relevant since a larger collaboration study with Western University in Canada is being launched instead.
Milestone name: Get IRB approval for a clinical study
Milestone name: Design a revised prototype on paper and in CAD
Planned Date: 03/17/2017
Expected Date: 05/15/2017
Status: Resolved. Mentor has voluntarily assumed responsibility for developing the new mechanical features of the device (i.e. brace attachment, finger cup attachment, and thumb silicone finger cup mold). The brace attachment is fully assembled; finger cup attachment mechanism and silicone cup mold have been fabricated off-site and are currently being shipped. My main responsibility is designing and fabricating the PCB DAQ board. I have completed the schematic, and the 2-layer and subsequent 4-layer revision trace/component layouts. According to the design rule checker on my software and at the chosen PCB fabricator, my design does not violate any design rules and has been validated. Current goal is to contact fabricator to get an assembly quote and to have the design fabricated.
Milestone name: Initial study feedback
Milestone name: Fabricate revised design
Planned Date: 04/14/2017
Expected Date: 05/20/2017
Status: Unresolved. The breadboard prototype has been validated for 20 channel DAQ, and the PCB design has been validated for meeting design rules specified by our chosen fabricator. Current goal is to get an assembly quote and to have the board fabricated and shipped.
Milestone name: Get approval stamp from CES for revised prototype
Planned Date: 04/21/2017
Expected Date: Summer (08/2017)
Status: Unresolved. However, my personal contribution to a previously-planned CES report is complete and can be used in a new report.
Milestone name: Get IRB approval for a clinical study of the revised prototype
Milestone name: Revised prototype study feedback
Reports and presentations
Project Plan
Project Background Reading
Project Checkpoint
Paper Seminar Presentations
Project Final Presentation
Project Final Report
Project Bibliography
[1] K. Nagata, “Fingertip-mounted six-axis force sensor”. US Patent 6622575 B1, 7 July 1999.
[2] J. Xu, A. Haith, J. Krakauer, “Motor control of the hand before and after stroke,” Clinical Systems Neuroscience, Ed. K. Kansaku et al. Springer Japan, 2015.
[3] J. Xu, N. Ejaz, B. Hertler, M. Branscheildt, M. Widmer, A. Faria, M. Harran, J. Cortes, N. Kim, P. Celnik, T. Kitago, A. Luft, J. Krakauer and J. Diedrichsen, “Recovery of hand function after stroke: separable systems for finger strength and control,” bioRxiv, 2016.
[4] R. Pozos and J. Agraz, “Force measuring device and method”. US Patent 6673026 B2, 27 March 2000.
[5] S. Ito, H. Kawasaki, Y. Ishigure, Y. Nishimoto, T. Aoki, T. Mouri, H. Sakaeda and M. Abe, “Development of a Hand Motion Assist Robot for Rehabilitation Therapy by Patient Self-Motion Control,” in Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics, Noordwijk, Netherlands, 2007.
Other Resources and Project Files