Table of Contents
Implementation of Body Surface Potential Map of QRST interval
— smurthy9 2012/05/10 13:13
Summary
- Students: Sindhoora Murthy and Markus Kowalsky
- Mentor(s): Dr. Larisa Tereschenko, Dr. Al Lardo, and Dr. Fady Dawoud
Background, Specific Aims, and Significance
Specific Aims:
Combine Sum Absolute Integral (SAI) QRST and Body Surface Potential Mapping (BSPM) to provide a better way to predict ventricular arrhythmias
Potential Applications:
- Used to determine whether or not a Cardiac Resynchronization Therapy is appropriate for a patient.
- Non-invasive mapping of a newly realized marker of cardiac disease.
- Provide greater prognostic and diagnostic information to electrophysiologists.
Background:
Approximately 350,000 people die of sudden cardiac death every year in the United States [1]. Sudden deaths are responsible for half of the deaths related to cardiovascular disease, and are primarily caused by Ventricular Tachyarrhythmia or Ventricular Fibrillation (VT or VF), which is rapid or abnormal contraction of the ventricles [2]. To diagnose these patients, doctors use ECGs to try and understand what is happening within the heart’s conduction systems.
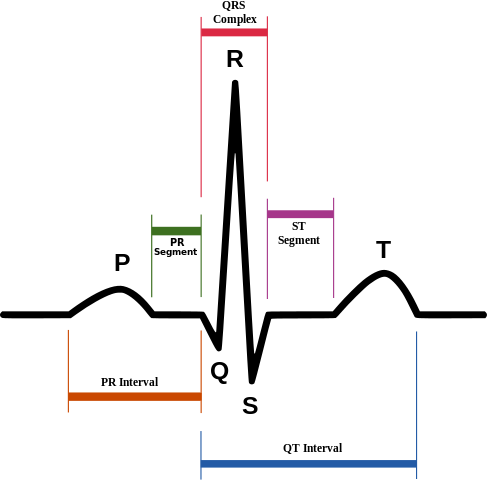
Normal ECG waveforms (the QRST regions are shown below as well):
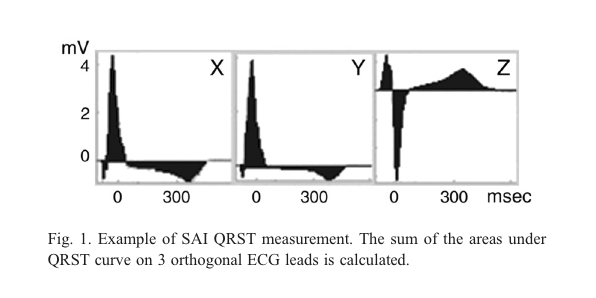
It has been shown in large group studies that SAI QRST can be used to predict ventricular arrhythmia in 12-lead electrocardiograms [3]. The figure below shows a SAI QRST calculated from an orthogonal ECG (different from a body surface ECG) [3]:
VT and VF are highly lethal conditions and once diagnosed, require immediate attention. In order to do so, it would be far more useful to preoperatively determine electrical maps of patients rather than intraoperatively, which carries far greater risks. Furthermore, work has not been done to combine SAI QRST with BSPMs, which will likely have a tremendous, non-invasive prognostic value. This method of joining SAI QRST with body surface mapping would allow for the screening of the general population at risk of sudden cardiac death.
BSPMs, particularly when used in conjunction with other imaging modalities such as CT or MRI, known as Electrocardiographic Imaging or ECGi, have much better prognostic value over conventional potential maps generated by catheter-fed electrodes [2].
One final motivation for the use of SAI QRST is to determine whether or not cardiac resynchronization therapy (CRT) will be successful. Currently around one third of patients do not improve with CRT but recent studies have shown that patients with longer QRS widths are more likely to respond positively to CRT while those with shorter QRS intervals are not [4]. We believe that by using SAI QRST we will be able to figure out whether a patient will respond well to CRT or whether other options should be considered.
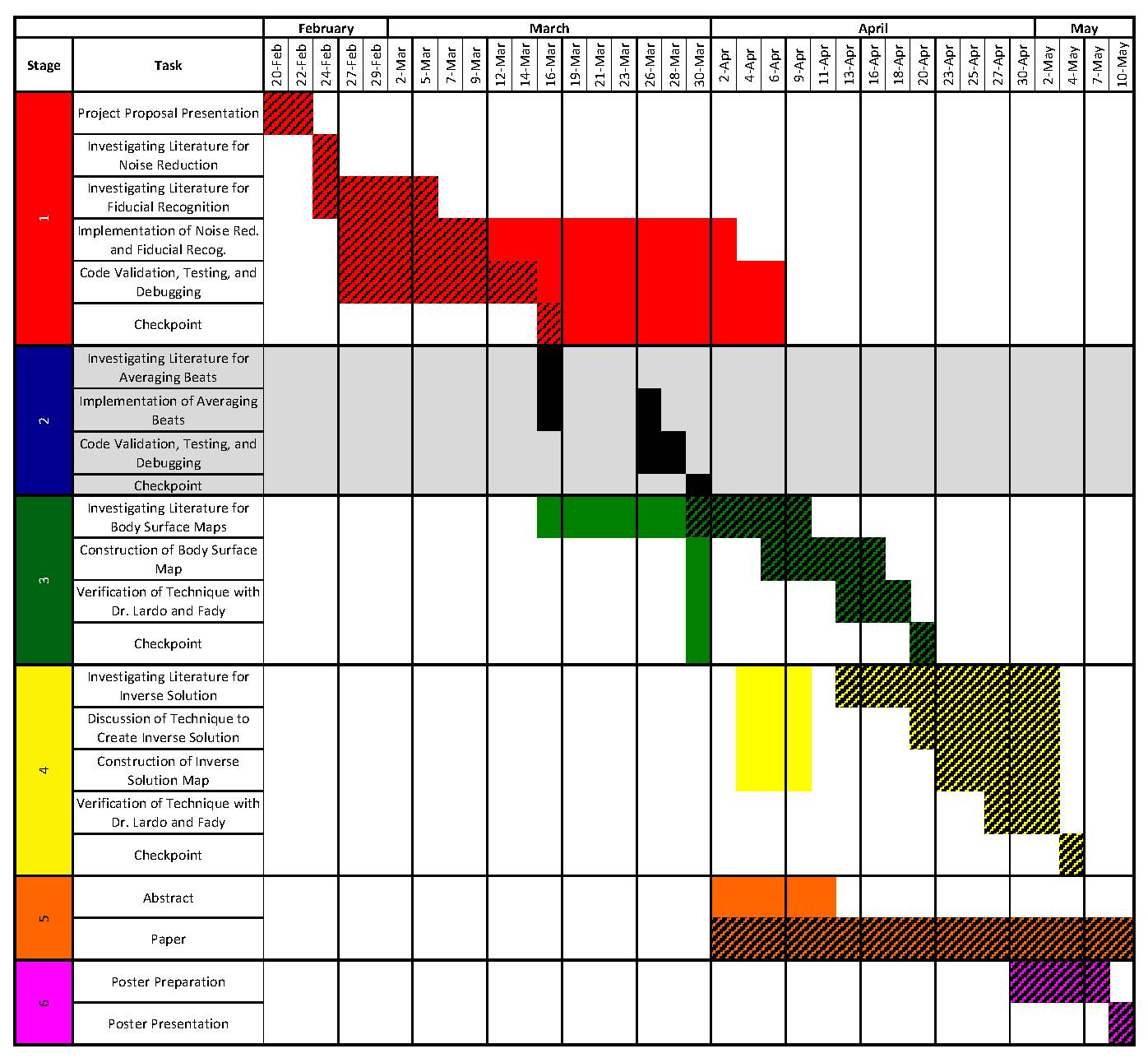
Deliverables
- Minimum: (Expected by March 30)
- Semi-automatically pre-processing 120-lead ECG data
- Automatically detecting fiducial points
- Calculating the sum absolute QRST integral
- Averaging the sum absolute QRST integral for each lead
- Expected: (Expected by April 20)
- Semi-automatically pre-processing 120-lead ECG data
- Automatically detecting fiducial points
- Calculating the sum absolute QRST integral
- Averaging the sum absolute QRST integral for each lead
- Constructing body surface map of sum absolute QRST integral
- Maximum: (Expected by May 4)
- Semi-automatically pre-processing 120-lead ECG data
- Automatically detecting fiducial points
- Calculating the sum absolute QRST integral
- Averaging the sum absolute QRST integral for each lead
- Constructing body surface map of sum absolute QRST integral
- Constructing map of the heart via the inverse solution
Technical Approach
The basic approach behind our project will involve four main stages:
- Pre-processing 120-lead ECG data
- Averaging data
- Constructing Body Surface Map
- Constructing Inverse Heart Map
Stage 1: Pre-processing Data
In this stage, we will rely heavily on previous work for detecting ECG fiducial points. Specifically, we plan on using work by Zong, et. al [5,6] to detect the time of PQ junction and end of the T wave. Using these time points, calculation of the sum absolute and native integrals of the QRST interval is fairly basic.
A brief outline for calculating the time points listed above is shown below as well as a quick visual summary of the first steps [6].
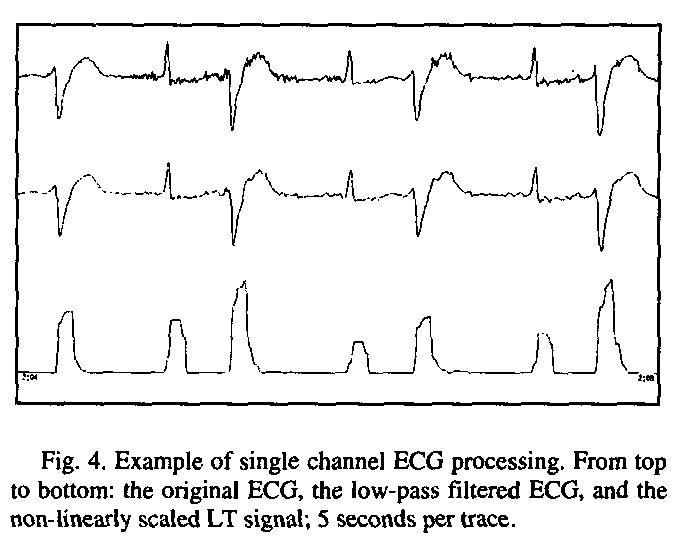
PQ junction detection
- Low pass filter for removing noise
- Curve length transformation over a time window equal to longest QRS complex (roughly 160ms)
- Decisions as to when curves begin and end
Automatic lead detection
- Select the lead with highest T-wave deflection
T-wave end detection
- Low pass filter out the noise
- Set entire PQRS region to value immediately prior to PQRS region
- Flip the signal horizontally
- Apply same method as PQ junction detection
Stage 2: Averaging Leads
Having calculated the sum absolute and native integrals of the QRST interval, we will then calculate the averaged beat across each of the 120 leads.
Our mentor had already aligned and averaged the data, so this step was not necessary.
— smurthy9 2012/04/03 21:35
Stage 3: Body Surface Map Construction
This stage will involve previously implemented techniques for calculating body surface potential maps from 120 lead ECG.
Markus used simple patch commands to construct a body surface map using Matlab's patch commands. — smurthy9 2012/04/03 21:36
Stage 4: Inverse Heart Map Construction
This stage will involve previously implemented techniques for calculating the inverse solution from 120 lead ECG data.
Inverse heart projection was computed with the help of our mentor, Dr. Fady Dawoud.
The epicardial maps serve to provide better spatial resolution of intercardiac events. — smurthy9 2012/04/03 21:37
Dependencies
IRB Approval
- Mentors need IRB approval to release data
- Status: Resolved
Data Source
- See above
- Status: Resolved
Weekly support meetings with Dr. Tereshchenko
- Assistance with first two stages of project
- Status: Resolved
Packages to help solve the inverse problem and create body surface and heart maps
- When we reach Stage 3 (projected March 20), we can acquire these from Fady
- Status: Resolved
Meetings with Dr. Lardo or Fady for help with constructing body surface and heart maps
- Fady (Dr. Lardo’s PhD student) will be primary contact and provide assistance with constructing these maps
- Status: Resolved
Milestones and Status
- Milestone name: Stage 1
- Planned Date: 3/16/12
- Expected Date: 3/16/12
- Status: Active
- Milestone name: Stage 3
- Planned Date: 4/20/12
- Expected Date: 4/20/12
- Status: Completed.
- Milestone name: Stage 4
- Planned Date: 5/4/12
- Expected Date: 5/4/12
- Status: Completed. Will work on more data.
- Milestone name: Abstract
- Planned Date: 4/9/12
- Expected Date: 4/9/12
- Status: Submitted
- Milestone name: Paper
- Planned Date: Early June
- Expected Date: Early June
- Status: Pending additional data.
Stage 2 is no longer relevant.
Reports and presentations
- Project Plan
- Project Background Reading
- See Bibliography below for links.
- Project Checkpoint
- Paper Seminar Presentations
- Project Final Presentation
- Project Final Report
- Source code at bottom of page.
Project Bibliography
References:
[1] Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009;119:480.
[2] Wang Y, Cuculich PS, Zhang J, Desouza KA, Smith TW, Rudy Y. Noninvasive Electroanatomic Mapping of Human Ventricular Arrhythmias with Electrocardiographic Imaging (ECGI). Science Translational Medcine 2011;84.
[3] Tereshchenko LG, Cheng A, Fetics BJ, et al. A new electrocardiogram marker to identify patients at low risk for ventricular tachyarrhythmias : sum magnitude of the absolute. Journal of Electrocardiology 2011;44(2):208-216.
[4] Ghosh S, Silva JN a, Canham RM, et al. Electrophysiologic substrate and intraventricular left ventricular dyssynchrony in nonischemic heart failure patients undergoing cardiac resynchronization therapy. Heart rhythm : the official journal of the Heart Rhythm Society 2011;8(5):692-9.
[5] Zong W, Saeed M, Heldt T, America N, Manor B. A QT Interval Detection Algorithm Based on ECG Curve Length Transform Materials and methods. Computers in Cardiology 2006:377-380.
[6] Zong W, Moody B, Jiang D. A Robust Open-source Algorithm to Detect Onset and Duration of QRS Complexes. Computers in Cardiology 2003;30:737-740.
Reading List:
1. Ghosh S, Silva JN a, Canham RM, et al. Electrophysiologic substrate and intraventricular left ventricular dyssynchrony in nonischemic heart failure patients undergoing cardiac resynchronization therapy. Heart rhythm : the official journal of the Heart Rhythm Society 2011;8(5):692-9.
2. Ambroggi LD, Corlan AD. Body Surface Potential Mapping. In: Comprehensive Electrocardiology., 2011:1376-1413.
3. Rudy Y. Cardiac repolarization : Insights from mathematical modeling and electrocardiographic imaging ( ECGI ). HRTHM 2009;6(11):S49-S55.
4. Wang Y, Cuculich PS, Zhang J, Desouza KA, Smith TW, Rudy Y. Noninvasive Electroanatomic Mapping of Human Ventricular Arrhythmias with Electrocardiographic Imaging ( ECGI ). 2011;84.
5. Tereshchenko LG, Cheng A, Fetics BJ, et al. A new electrocardiogram marker to identify patients at low risk for ventricular tachyarrhythmias : sum magnitude of the absolute. Journal of Electrocardiology 2011;44(2):208-216.
6. Tereshchenko LG, Cheng A, Fetics BJ, et al. Ventricular arrhythmia is predicted by sum absolute QRST integral but not by QRS width. Journal of Electrocardiology 2010;43(6):548-552.
7. Sornmo L, Laguna P. ELECTROCARDIOGRAM (ECG) SIGNAL PROCESSING. Wiley Encyclopedia of Biomedical Engineering 2006:1-16.
8. Zong W, Saeed M, Heldt T, America N, Manor B. A QT Interval Detection Algorithm Based on ECG Curve Length Transform Materials and methods. Computers in Cardiology 2006:377-380.
9. Zong W, Moody B, Jiang D. A Robust Open-source Algorithm to Detect Onset and Duration of QRS Complexes. Computers in Cardiology 2003;30:737-740.