Table of Contents
Intra-operative OCT Assesment
Last updated: 02/15/12 at 12:16 PM
Summary
We aim to assess the efficacy of intra-operative OCT (Optical Coherence Tomography) imaging as an aid in vitrioretinal surgery, in particular for peeling epiretinal membranes (ERMs). We intend to investigate this using a simulated micro-surgical task. Secondarily, we seek to improve the existing user interface of the OCT system in order to advance the system towards potential clinical use.
- Students: Andrea Corredor, Amrita Gupta
- Mentor(s): Marcin Balicki
Background, Specific Aims, and Significance
Background
Optical Coherence Tomography
OCT provides micron-scale images of anatomical structures within a given tissue. Broadband light passes down a single optical fiber, gets reflected by tissue layers and interacts with itself. The reflected light is fed to a spectrometer, which performs Fourier analysis to return depth information on the anatomical features. The depth image at a single point is an Axial or A-mode image. Taking a continuous scan and combining the sequence of A-mode images produces a distorted cross-sectional B-mode scans.
Fiber Integrated Surgical Pick
By incorporating sensors directly into ophthalmic instruments it is possible to assess the structures ahead of or in contact with the instrument. In the case of the fiber-integrated surgical pick, surgeons can image using the same tool that they would use to remove ERMs, instead of changing tools and re-imaging frequently.
Visual Tracking and Annotation
The stereo video microscope is registered with the position of the intraoperative OCT imaging tool, and the video feed is annotated with the paths of the tool scans. This enables surgeons to return to locations on the retina that show anatomical features of interest in the OCT scan.
Integration with Assistive Robots
While the OCT imaging pick can be used freehand by providing, for instance, auditory feedback to the surgeon, it can also be used as imaging feedback for active assistant robot like the SteadyHand. This presents many benefits like reduced hand tremor and increased accuracy (through movement attenuation and scaling).
Specific Aims
- Build a phantom
- Design a micro-surgical task
- Obtain IRB approval for task
- Conduct subject trials
- Perform statistical analysis of data from experiments
- Overlay OCT mScan paths on stereocamera view of operating area
- Implement mScan dewarping
Motivation
Vitreoretinal surgery is technically demanding for surgeons, and is made especially challenging due to factors such as: - difficulty visualizing surgical targets - small size and delicate nature of tissues - voluntary and involuntary movement of the patient’s eye - hand tremor and lack of force feedback
The OCT-integrated pick paired with the JHU EyeRobot platform attempts to address many of these factors. Specifically, the OCT system can be used to provide high-resolution spatial information and tissue depth perception. The driving application of this feature is the ability to locate the edges of epiretinal membranes for removal. These are very small delicate membranes that need to peeled from the retina, but are clear and very difficult to locate. Intra-operative OCT imaging can greatly facilitate the localization of these membranes, and incorporation of OCT imaging with an assistive robot can address issues like accuracy, hand tremor and safety.
Deliverables
- Minimum: (Expected by 04/16/2012)
- Phantom
- IRB approval
- Subject experiment
- Robot Integration
- Expected: (Expected by 04/23/2012)
- Time-space differences correction
- Results from executed experiments
- Statistical analysis of results
- OCT image enhancement
- Maximum: (Expected by 05/07/2012)
- Functional demo of GUI
- Report
- Refined m-scan user interface
Technical Approach
Experimental Validation of mScan for Locating ERM Edges
Phantom Design
In order to test the efficacy of the mScan-OCT system for detecting ERM edges, there is the need for a realistic phantom eye, in which the retina surface has regions of scar tissue. In particular, this scar tissue must have the following properties: * transparent * non-reflective * thin
The ERM regions should be e↵ectively invisible to the naked eye, and also difficult to locate with stereomicroscopy, which could be achieved by the use of a material with similar or identical properties to he material of which the retina surface is made.
Experimental Task Design and Data Analysis
The purpose of the experimental task is to evaluate whether using OCTsystem-generated mScans improves the success rate of finding ERM edges with the properties described above. In order to simulate intraoperative conditions as closely as possible, we will attend vitreoretinal surgeries on both humans and on rabbits to better appreciate the challenges faced by surgeons. We will also consult them for suggestions on the type of tasks our experiment could include. This may involve supplying participants with a radial pattern of ”pre-operative” mScans from the test retinas, instructions to find as many edges within an allocated time, progressively more difficult retina samples, and/or the use of the EyeRobot in conjunction with the OCT probe versus freehand operation of the probe. The statistical significance of the outcome will be assessed using a test suitable to the specific experimental design, the number of trials and study participants.
GUI and User Interface
The existing OCT system returns the position of the OCT probe tip versus time, while the mScan contains depth information versus time. By combining the information contained in these two modalities, a feature could plausibly be developed in which an interesting section of an mScan can be mapped to a position in stereovideo. The visual rendering of this information in the operating view will be improved, taking into account the feedback from the eye surgeons we will consult. We will also investigate encoding an automatic scan of the retina, providing high-resolution OCT information over a small region of tissue.
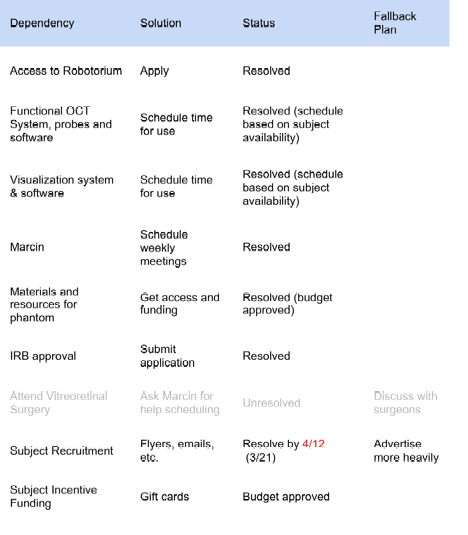
Dependencies
Milestones and Status
- Milestone name: Design of micro-surgical task that simulates ERM peeling
- Planned Date: 3/12/12
- Expected Date: 3/21/12
- Status: Done!
- Milestone name: Working phantom
- Planned Date: 3/12/12
- Expected Date: 4/1/12
- Status: Done!
- Milestone name: IRB approval
- Planned Date: 3/19/12
- Expected Date: 4/4/12
- Status: Done!
- Milestone name: Completion of advertisement and incentive for subject recruitment
- Planned Date: 3/19/12
- Expected Date: 4/11/12
- Status: Done!
- Milestone name: Completion subject trials
- Planned Date: 4/16/12
- Expected Date:6/25/12
- Status: In progress: to be continued
- Milestone name: Statistical analysis of data from subject trials
- Planned Date: 4/16/12
- Expected Date:6/30/12
- Status: In progress: to be continued
- Milestone name: OCT enhancements (Color enhancements, GUI improvements)
- Planned Date: 4/9/12
- Expected Date: 7/15/12
- Status: Not yet begun, relegated to future work
- Milestone name: Implementation of time-space mScan correction
- Planned Date: 4/9/12
- Expected Date: 5/7/12
- Status: Done!
Reports and presentations
- Project Plan
- Project Background Reading
- See Bibliography below for links.
- Project Checkpoint
- Paper Seminar Presentations
- Project Final Presentation
- Project Final Report
Project Bibliography
* “Single Fiber Optical Coherence Tomography Microsurgical Instruments for Computer and Robot-Assisted Retinal Surgery” Marcin Balicki, Jae-Ho Han, Iulian Iordachita, Peter Gehlbach, James Handa, Jin Kang, Russell Taylor.
* “Common-path Fourier-domain Optical Coherence Tomography with a Fiber Optic Probe Integrated Into a Surgical Needle” Jae-Ho Han, Marcin Balicki, Kang Zhang, Jae-Ho Han, Marcin Balicki, Kang Zhang, Xuan Liu, James Handa, Russell Taylor, and Jin U. Kang; Proceedings of CLEO Conference, May 2009
* “Micro-Force Sensing in Robot Assisted Membrane Peeling for Vitreoretinal Surgery” Marcin Balicki, Ali Uneri1, Iulian Iordachita, James Handa, Peter Gehlbach, Russell Taylor. Proceedings of the MICCAI Conference, 2010.
* “Automatic online spectral calibration of Fourier-domain OCT for robot-assisted vitreoretinal surgery” Xuan Liu, Marcin Balicki, Russell H. Taylor, and Jin U. Kang. , in SPIE Advanced Biomedical and Clinical Diagnostic Systems IX,25 January 2011.
* “Augmented Reality Fundus Biomicroscopy. A Working Clinical Prototype.” Je↵rey W. Berger, MD, PhD; Bojidar Madjarov, MD. Arch Ophthalmol. 2001
* “Biopsy site re-localisation based on the computation of epipolar lines from two previous endoscopic images.” Allain B, Hu M, Lovat LB, Cook R, Ourselin S, Hawkes D. Centre for Medical Image Computing, University College London
* “Optical biopsy mapping for minimally invasive cancer screening.” Peter Mountney, Stamatia Giannarou, Daniel Elson, Guang-Zhong Yang. Department of Computing, Imperial College, London SW7 2BZ, UK. MICCAI International Conference on Medical Image Computing and Computer-Assisted Intervention 01/2009; 12(Pt 1):483-90.