Table of Contents
Robotically Assisted Cochlear Imaging and Access
Last updated: 05/10/2012 and 12:00
Summary
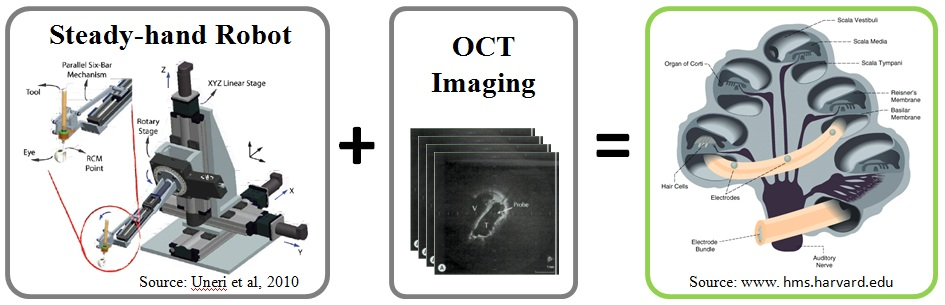
Cochlear implants are electronic devices that are used to restore hearing to individuals with severe-to-profound sensorineural loss via direct stimulation of the auditory nerve. Implantation requires the insertion of an electrode into human cochlea. This project aims at developing a safe robotic approach for minimizing intracochlear trauma during electrode insertion and consequently preserving the natural residual hearing in patients. For this purpose, OCT-imaging techniques will be integrated with the steady-hand robot. By imaging and modeling the cochlea, and eliminating hand-tremor, the system will assist in placing a cochlear implant electrode array in the right position with no intracochlear trauma.
- Students: Ehsan Azimi,Berk Gonenc
- Mentor(s): R. Taylor, I. Iordachita, J. Kang, J. Niparko, W. Chien
Background, Specific Aims, and Significance
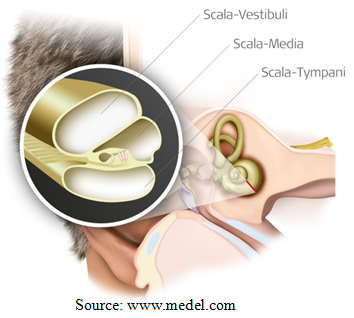
Cochlear implants aim at direct stimulation of the auditory nerve via an electrode array placed inside the cochlea. The state of the art for this operation is a facial recess approach to the middle ear. After the cochleostomy is performed by recess, the array is manually inserted into the scala tympani using special tools such as claws and alligator forceps. Scala tympani is separated from the other two fluid filled compartments inside the cochlea only by a thin basilar membrane. Basilar membrane is a very delicate layer, which accommodates the inner hairy cells that generate the neural activities associated with hearing. Hence any damage on this layer will cause hearing loss or deafness.
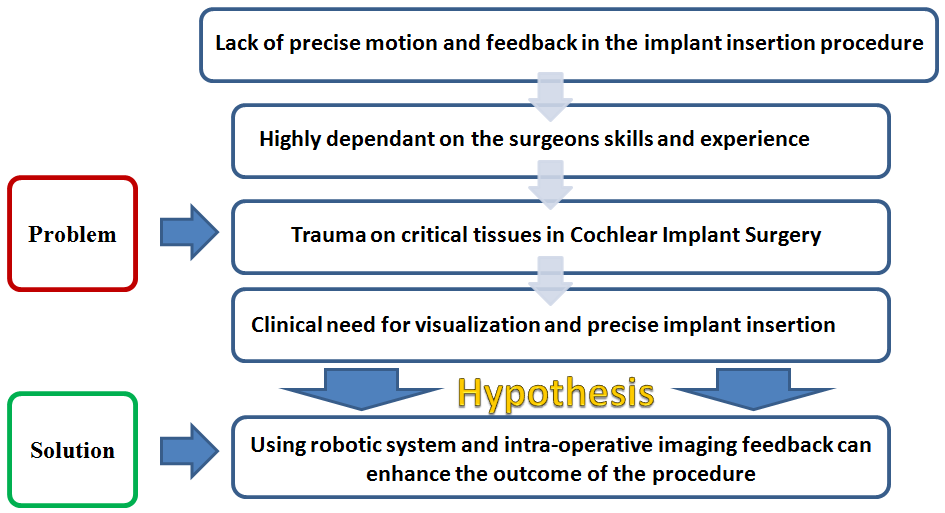
Combined electrical and acoustic stimulation is a new technique to restore hearing to patients with severe hearing loss, but still with some residual hearing, by amplifying the residual hearing via a hearing aid and simultaneously stimulating the cochlear nerve via an implant. For this strategy, it is very important to preserve the patient's residual hearing during implant surgery. However, using the conventional manual methods, most of this residual hearing is often lost due to various operatively caused traumas [1-10]. Using a robotic system with intra-operative imaging feedback can significantly enhance the outcome of the procedure by preventing such traumas.
In the cochlear implant surgery, a flexible curved electrode array (1 mm diameter) is advanced into 15-20 mm long cochlea channel. During insertion, “Advance Off-Stylet Technique” is used, which consists of 3 fundamental steps:
1. The whole electrode is inserted until the white marker reaches cochleostomy site. This corresponds to an insertion depth of approximately 7 mm. 2. The stylet is held stationary, electrode is deployed off the stylet. The electrode takes its naturally curved shape. 3. After the ribs reach the cochleostomy site, the stylet is removed.
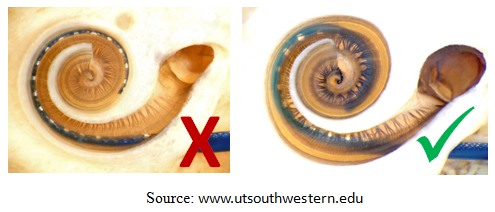
For a successfull surgery, the location of each step is very critical. Failure to follow the presented steps during the surgery can result in intracochlear trauma and incorrect placement of the electrode. Factors such as poor visualization and physiological hand tremor of the surgeon make the procedure quite challenging and limit the success of the surgery.
Clinical Goals:
1. Among the three channels inside cochlea, the electrode should travel only in Scala-Tympani with no damage to the basilar membrane.
2. The electrode should be located as close to modiolus as possible for better stimualtion of the hearing nerve.
Project Goals:
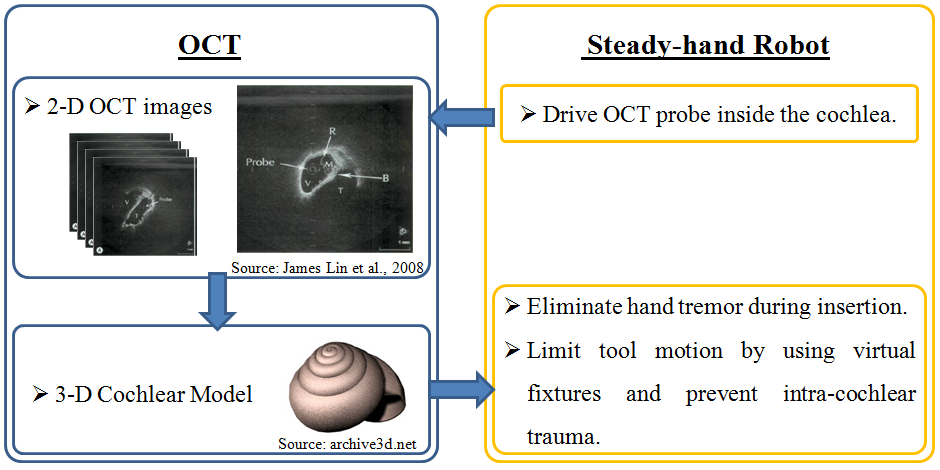
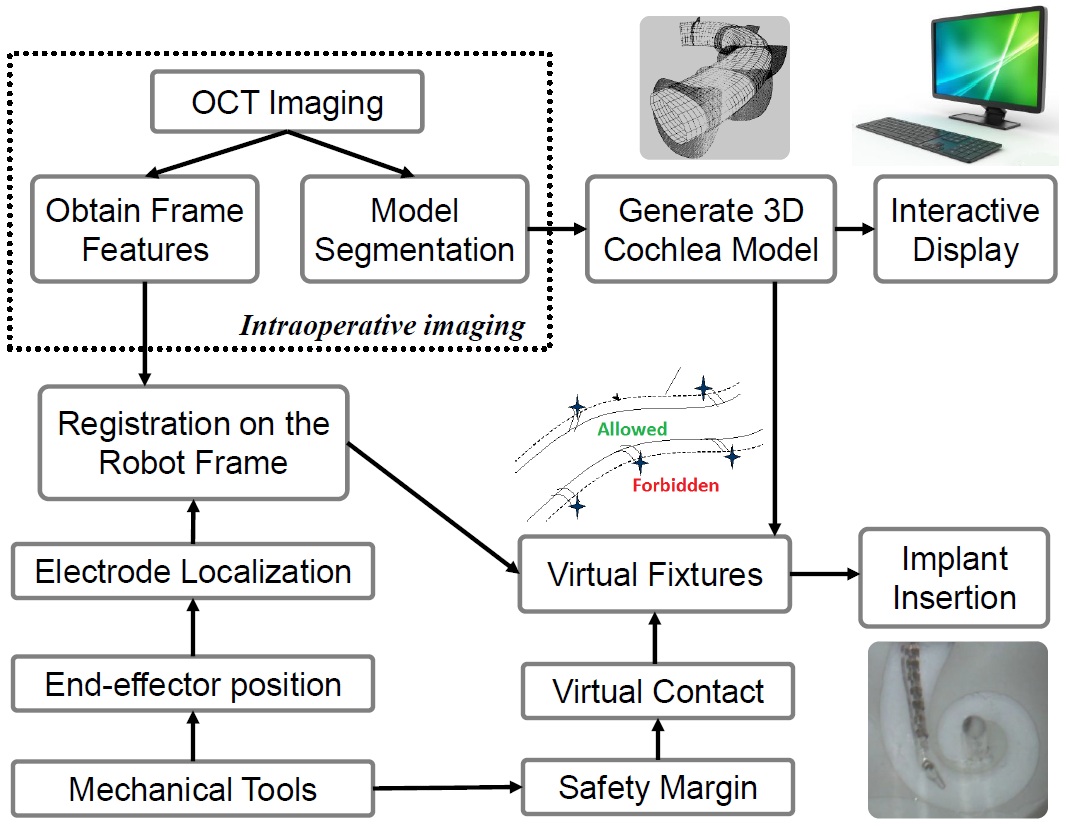
1. Use Optical Coherence Tomography (OCT) to build 3-D cochlea model.
2. Use Steady-hand Robot to eliminate hand-tremor.
3. Implement virtual fixtures for use with the robot for optimal electrode placement.
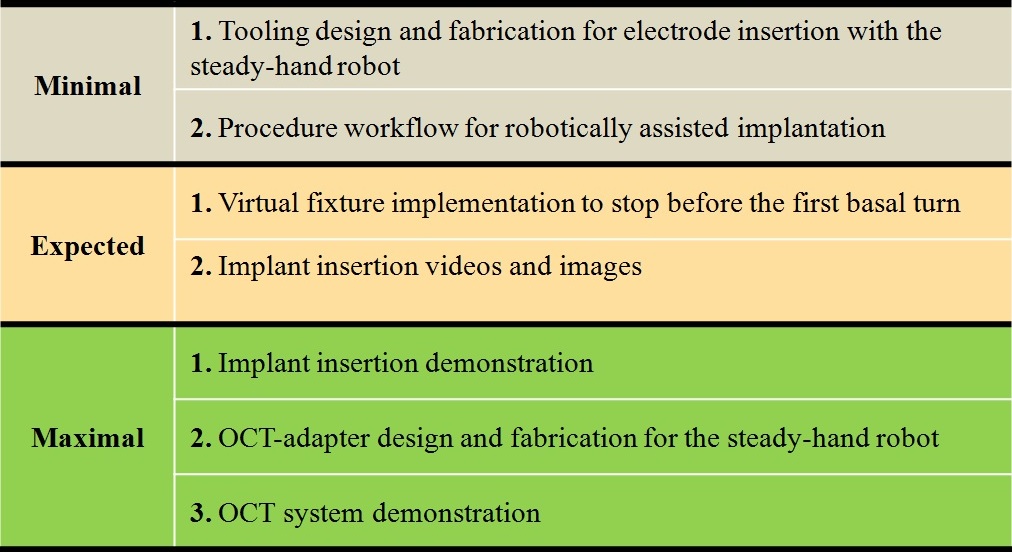
Deliverables
Technical Approach
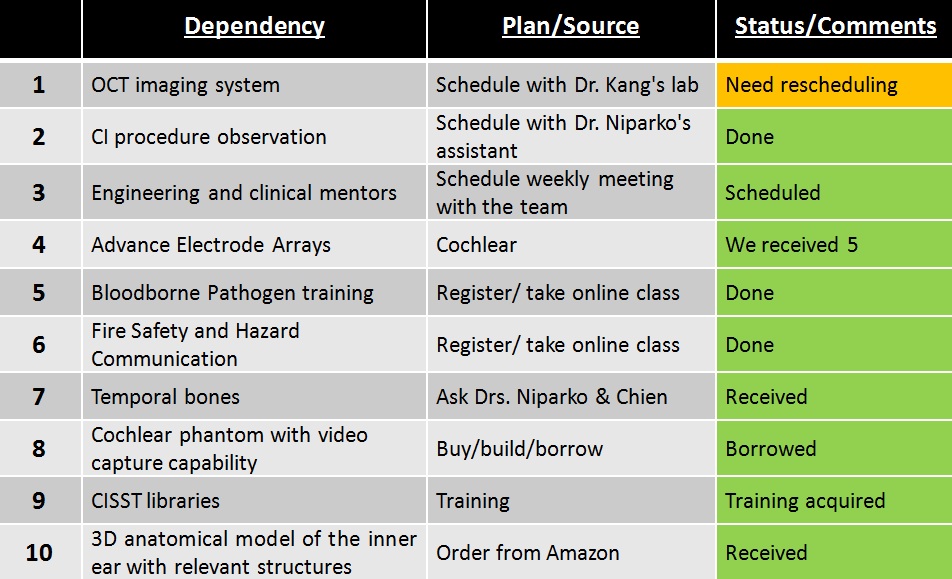
Dependencies
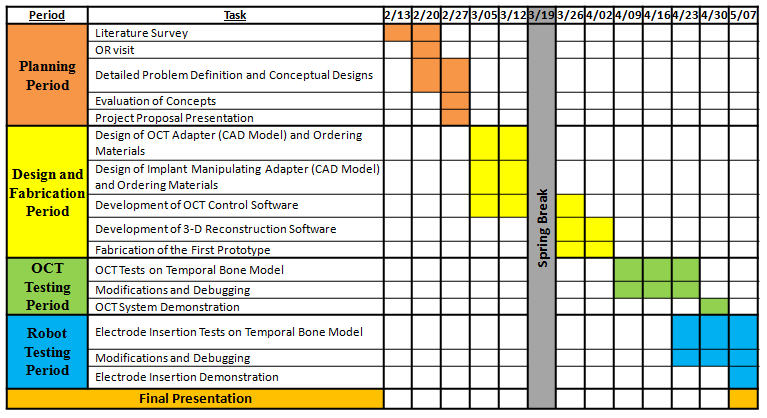
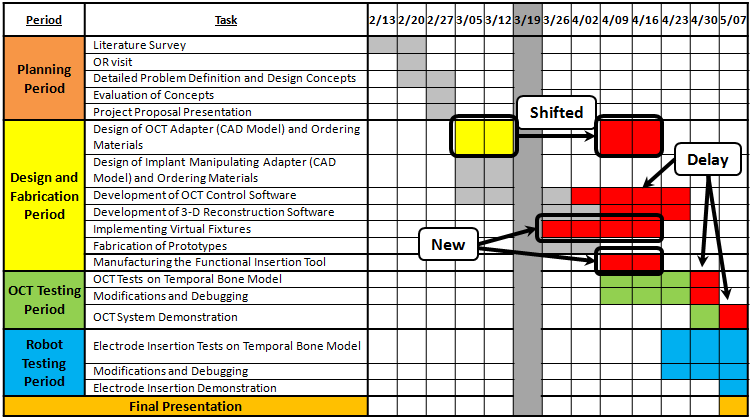
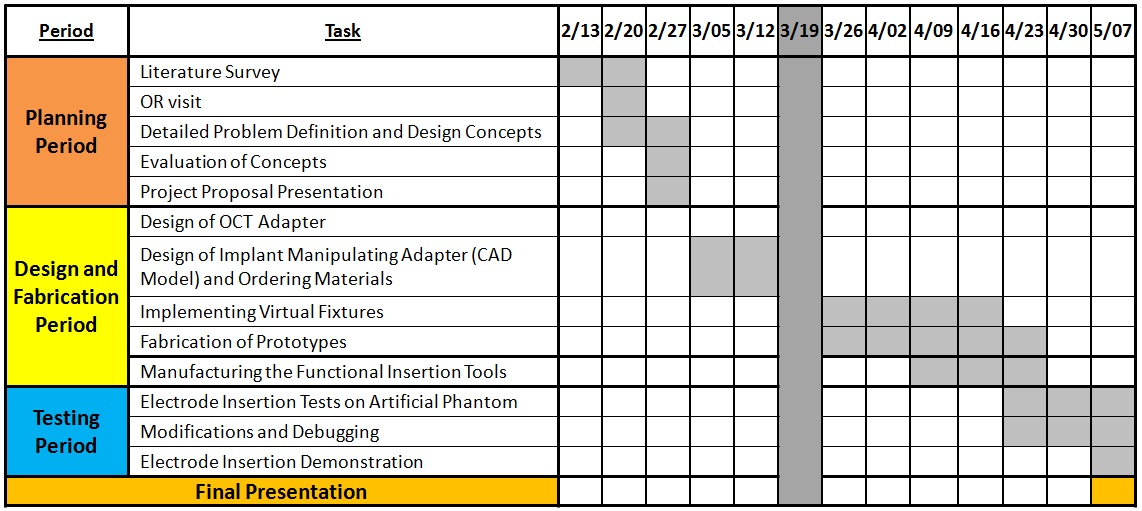
Milestones and Status
Reports and presentations
- Project Plan
- Project Background Reading
- See Bibliography below for links.
- Project Checkpoint
- Paper Seminar Presentations
- Project Final Presentation
- Project Final Report
- links to any appendices or other material
Project Bibliography
[1] Lenarz T, Stöver T, Buechner A et al (2006) Temporal bone results and hearing preservation with a new straight electrode. Audiol Neurootol 11(Suppl 1):34–41.
[2] Roland P, GstöttnerW,AdunkaO (2005) Method for hearing preservation in cochlear implant surgery. OperativeTech 16(2):93–100.
[3] AdunkaOF, Pillsbury HC,Kiefer J (2006) Combining perimodiolar electrode placement and atraumatic insertion properties in cochlear implantation—fact or fantasy? Acta Otolaryngol 126(5):475–482.
[4] Adunka OF, Radeloff A, Gstoettner WK et al (2007) Scala tympani cochleostomy. II. Topography and histology. Laryngoscope 117(12):2195–2200.
[5] Briggs RJS, Tykocinski M, Stidham K et al (2005) Cochleostomy site: implications for electrode placement and hearingbpreservation. Acta Otolaryngol 125(8):870–876.
[6] Eshraghi AA, Yang NW, Balkany TJ (2003) Comparative study of cochlear damage with three perimodiolar electrode designs. Laryngoscope 113(3):415–419.
[7] Roland PS,WrightCG (2006) Surgical aspects of cochlear implantation: mechanisms of insertional trauma. Adv Otorhinolaryngol 64:11–30.
[8] Stöver T, Issing P, Graurock G et al (2005) Evaluation of the advance off-stylet insertion technique and the cochlear insertion tool in temporal bones. Otol Neurotol 26(6):1161–1170.
[9] Wardrop P, Whinney D, Rebscher SJ et al (2003) A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. I. Comparison of Nucleus banded and Nucleus Contour electrodes. Hear Res 203(1–2):54–67.
[10] Wardrop P,Whinney D, Rebscher SJ et al (2005) A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. II. Comparison of Spiral Clarion and HiFocus II electrodes. Hear Res 203(1–2):68–79.
[11] Hussong A, Rau T, Ortmaier T et al (2010) An automated insertion tool for cochlear implants: another step towards atraumatic cochlear implant surgery. International Journal of Computer Assisted Radiology and Surgery 5:163–171.
[12] United States Patent 7184843: Electrode array with non-uniform electrode spacing.
[13] Cohen LT, Saunders E, Clark GM (2001) Psychophysics of a prototype peri-modiolar cochlear implant electrode array. Hear Res 155(1–2):63–81.
[14] Rau T, Hussong A, Lewinung M et al (2009) Automated insertion of preformed cochlear implant electrodes: evaluation of curling behavior and insertion forces on an artificial cochlear model. International Journal of Computer Assisted Radiology and Surgery 5:173–181.
[15] Hussong A, Rau T, Eilers H et al (2008) Conception and design of an automated insertion tool for cochlear implants. Engineering in Medicine and Biology Society. EMBS 2008. 30th Annual International Conference of the IEEE , pp.5593-5596, 20-25 Aug. 2008.
[16] Schurzig D, Labadie R, Hussong A, Rau T, Webster R (2010) A Force Sensing Automated Insertion Tool for Cochlear Electrode Implantation. IEEE International Conference on Robotics and Automation, May 2010.
[17] Schurzig D, Labadie R, Hussong A, Rau T, Webster R (2012) Design of a Tool Integrating Force Sensing With Automated Insertion in Cochlear Implantation. IEEE/ASME Transactions on Mechatronics, 17(2): 381-389.
[18] Kratchman L, Blachon G, Withrow T et al (2010) Toward Auutomation of Image-Guided Microstereotactic Frames: A bone-Attached Parallel Robot for Percutaneous Cochlear Implantation. Robotics Science and Systems 2010: Workshop on Enabling Technologies.
[19] Kratchman L, Blachon G, Withrow T et al (2011) Design of a Bone-Attached Parallel Robot for Percutaneous Cochlear Implantation. IEEE Transactions on Biomedical Engineering, 58(10):2904-2910.
[20] Thomas S. Rau, Omid Majdani, Andreas Hussong, Thomas Lenarz, Martin Leinung. Determination of the curling behavior of a preformed cochlear implant electrode array“, Int J CARS (2011) 6:421–433.
[21] James Lin, MD; Hinrich Staecker, MD, PhD; M. Samir Jafri, PhD “Optical Coherence Tomography Imaging of the Inner Ear: A Feasibility Study With Implications for Cochlear Implantation” Annah of Olülugy, Rhinology & Laryngohgy 117(5):341-346. 2008.
[22] HANS WILHELM PAU, EVA LANKENAU, TINO JUST, DETLEF BEHREND & GEREON HUTTMANN “Optical coherence tomography as an orientation guide in cochlear implant surgery?” Proc. of SPIE Vol. 6842 68421F-1.
[23] Costas Pitris, PhD; Kathleen T. Saunders, BS; James G. Fujimoto, PhD; Mark E. Brezinski, MD, PhD “High-Resolution Imaging of the Middle Ear With Optical Coherence Tomography: A Feasibility Study” ARCH OTOLARYNGOL HEAD NECK SURG/VOL 127, JUNE 2001.
[24] Hrebesh M. Subhash Viviana Davila et al.”Volumetric in vivo imaging of intracochlear microstructures in mice by high-speed spectral domain optical coherence tomography“ Journal of Biomedical Optics 15_3_, 036024 _May/June 2010.
[25] Daniel Schurzig, Zachariah W. Smith, D. Caleb Rucker, Robert F. Labadie, Robert J. Webster III, A manual insertion mechanism for percutaneous cochlear implantation, Design of Medical Devices Conference (DMD2010), April 13-15 2010, Minneapolis, MN, USA
[26] Ramya Balachandran, Jason E. Mitchell, Jack Noble, Daniel Schurzig, Gregoire Blachon, Theodore R. McRachan, Robert J. Webster, Benoit M. Dawant, J. Michael Fitzpatrick, Robert F. Labadie, Insertion of electrode array using percutaneous cochlear implantation technique: a cadaveric study, Medical Imaging 2011: Visualization, Image-Guided Procedures, and Modeling, February 13 2011, Lake Buena Vista, FL, USA
[27] Daniel Schurzig, Robert F. Labadie, Andreas Hussong, Thomas S, Rau, Robert J. Webster, A Force Sensing Automated Insertion Tool for Cochlear Electrode Implantation, IEEE International Conference on Robotics and Automation, May 2010
Other Resources and Project Files
Here give list of other project files (e.g., source code) associated with the project. If these are online give a link to an appropriate external repository or to uploaded media files under this name space.