Table of Contents
Ultrasound-Compatible Female Pelvic Phantom for Hydrogel Spacer Injection during Brachytherapy Training
Last updated: May 11, 2018
Summary
The goal of this project is to evaluate the feasibility of ultrasound needle guidance for hydrogel injection during cervical cancer brachytherapy. My role focuses on developing the prototype needle and training phantom for this specific application and conducting preliminary tests on the model.
- Students: Tracy Kao
- Mentor(s): Carmen Kut (MD/PhD); Emad M. Boctor, PhD; Akila Viswanathan, MD, MPH, M.Sc; Younsu Kim (PhD)
Background, Specific Aims, and Significance
Locally advanced cervical cancer is a common presentation among unscreened women. Unlike early-stage disease, locally advanced cervical cancer has finite survival times and cannot be cured by surgery alone (with a high relapse rate at 30%). Recent data have repeatedly and consistently shown the benefit of administering brachytherapy following external beam radiotherapy (EBRT) to prolong survival and to improve patient outcomes (when coupled with chemotherapy). However, brachytherapy is a complex, challenging procedure which requires accurate, real-time tracking and contouring of the cervical tumor mass to achieve maximal tumor control while maintaining minimal radiation toxicity to surrounding nearby structures e.g. the bladder and rectum. Thus, there is a clear need to differentiate the cervical tumor mass from surrounding normal tissues e.g. the rectovaginal septum during brachytherapy.
While other imaging technologies have been investigated (e.g. intraoperative MRI and preoperative MRI/CT), they are limited in terms of practicality and cost. This is especially a challenge in resource-limited countries which often harbor the highest rates of locally advanced disease (in part due to limited screening and unavailability of vaccines). For this project, we propose the use of advanced ultrasound imaging systems in addressing this important clinical problem.
In brachytherapy planning, it is routine practice to inject a hydrogel spacer to minimize radiation dose to normal anatomical structures. However, this is a challenging procedure, and inaccurate needle placement can lead to complications such as accidental perforation of the bowel and rectum. The project involves developing an advanced ultrasound needle guidance system which is initially designed for lumbar punctures. We hypothesize that advanced ultrasound imaging techniques can be applied to needle design for reliable and accurate guidance of needle placement into the recto-vaginal septum for hydrogel injections during brachytherapy.
There are two goals for the overaching project:
1. Adapt needle for ultrasound-compatibility in guidance for hydrogel spacer injection in the recto-vaginal septum.
2. Develop an ultrasound-compatible phantom to assist training on localizing and visualizing a needle for hydrogel space injection during the preparation of a patient for brachytherapy.
My scope, in terms of this course, is primarily focused on item 2, the construction of the phantom.
Deliverables
The deliverables listed here are the most updated version. For original deliverables, please reference the “Project Proposal” and “Project Presentation” files in a later section.
- Minimum: (Expected by 4/20/2018)
- Needle Design
- Phantom Design
- Expected: (Expected by 4/24/2018)
- Documentation: Needle Manufacturing and Testing Plan
- Documentation: Phantom Manufacturing and Testing Plan
- Simple Phantom (Geometric Shape, Realistic Texture)
- Maximum: (Expected by 5/10/2018)
- Simple Phantom (Geometric Shape, Ultrasound Compatible)
- Mold Used to Manufacture Phantom
- Documentation: Phantom Manufacturing and Test Results
Please see Documentation section below for links to the documentation described above.
Technical Approach
For this project, it is possible to take advantage of the research that has already been done at the Medical UltraSound Imaging and Intervention Collaboration (MUSiiC) Lab to address a clearly defined clinical need, particularly that of ultrasound guidance. The needle manufacturing process adapts the electronic and algorithmic structure of the Active Ultrasound Pattern Injection System (AUSPIS) for use with the hydrogen injection needle. In addition, this system will be attempted with a trans-rectal ultrasound system instead of with the standard abdominal ultrasound transducers, for optimal proximity to the site of interest.
My main goal for this course, however, is the creation of an ultrasound compatible phantom that can be used to for training residents or other medical professionals in hydrogel spacer injection in a delicate area, particularly the rectovaginal septum.
Multiple iterations making different shapes and hardness of the plastisol will be done prior to putting together the phantom. These tests will allow me to gain familiarity with the phantom making process.
My approach involves the use of plastisol, a long-lasting yet soft material that can be used to replicate the texture of tissue to make the phantom more realistic to the user. For the first phantom, I use gradations in the ratio of Soft Plastic to Plastic Softener to control the degree of softness of the tissue. I use geometric shapes to simulate the relevant organs and tissue structures, such as the vaginal wall, the uterus, and the rectal wall.
In the next step, I try to replicate the echogenicity of the female pelvis tissue by applying microbeads of varying densities to the plastisol. Different microbead distributions will create different acoustic patterns under ultrasound, and good control will allow us to replicate the ultrasound quality of the phantom, improving on existing models.
Finally, I expand on this work by using 3D prints from manual volume segmentation of patient MRI (with smoothing) on the 3D Slicer software in order to generate anatomically accurate molds for the phantom as an improvement on my second prototype.
As part of the evaluation, I use an abdominal ultrasound system and spinal needle to test the performance of these phantoms under ultrasound imaging, and evaluate whether they are useful for people practicing hydrogel injection in the brachytherapy procedure.
The complete detailed review of the technical approach and results, along with informative figures and tables, is available in the pdf below titled “Final Report.”
Dependencies
The dependencies listed here are the most updated version. For original dependencies, please reference the “Project Proposal” and “Project Presentation” files in a later section.
- Hydrogel Needle
- Deliverable Level: Expected
- Purpose: Used as a component in the needle design, and also as a means to test the phantom.
- Solution: Acquire from Clinical Consultant
- Important dates: 3/8/2018
- Alternatives: Use other needle.
- Status: Resolved as of 2/14/2018.
- Hardware Components
- Deliverable Level: Expected
- Purpose: Used to construct the adapted needle.
- Solution: Provision by MuSiiC Lab
- Important dates: 4/5/2018
- Alternatives: Place order as budget allows.
- Status: Partially resolved as of 2/14/2018. However, it was insufficient and will need to be delayed.
- Access to Lab Environment for Prototype Development
- Deliverable Level: Expected
- Purpose: In order to facilitate the construction of the adapted needle.
- Solution: Provision by MuSiiC Lab
- Important dates: 3/8/2018
- Alternatives: Use currently available lab space.
- Status: Resolved as of 3/30/2018
- Compatible Ultrasound System
- Deliverable Level: Minimum
- Purpose: For testing of our prototypes, both for the needle and for the phantom.
- Solution: Provision by MuSiiC Lab
- Important dates: 4/5/2018
- Alternatives: Use abdominal systems available at Simulation Centers at the Johns Hopkins Hospital.
- Status: Resolved as of 3/30/2018.
- Phantom Construction Materials (e.g. Plastisol, Microbead, Silicone, Metal, and Glass Containers)
- Deliverable Level: Expected, Maximum
- Purpose: In order to build the phantom, an essential part of the project.
- Solution: Use the materials available in lab space and purchase the remainder as necessary.
- Important dates: 4/20/2018
- Alternatives: Purchase materials as budget allows. Minimize complexity of phantoms.
- Status: Resolved as of 4/14/2018.
- Phantom Manufacturing Environment (Lab, Space, Hood)
- Deliverable Level: Expected, Maximum
- Purpose: In order to work with the chemicals that are used to construct the phantom. Safety concerns.
- Solution: Use the BME Design Studio in Clark Hall.
- Important dates: 4/20/2018
- Alternatives: Work outdoors.
- Status: Resolved as of 3/30/2018
- Phantom Manufacturing Equipment (3D Printing)
- Deliverable Level: Expected, Maximum
- Purpose: In order to facilitate the process of constructing the phantom. Good manufacturing equipment allows for more accurate and clean results in making phantom parts.
- Solution: Use the BME Design Studio in Clark Hall.
- Important dates: 4/20/2018
- Alternatives: Use Wyman Park Bldg. equipment.
- Status: Resolved as of 4/11/2018
Milestones and Status
The milestones and timeline listed here are the most updated version. For the original projected milestones and timeline, please reference the “Project Proposal” and “Project Presentation” files in a later section.
- Milestone name: Clinical Observation & Clinical Need Evaluation
- Level: Minimum
- Date: 3/1/2018
- Status: Complete
- Milestone name: Discussed Project Goals with All Mentors
- Level: Minimum
- Date: 3/6/2018
- Status: Complete
- Milestone name: Documentation: Needle Design and Specifications
- Level: Minimum
- Date: 3/8/2018
- Status: Complete, Available on Wiki
- Milestone name: Documentation: Needle Manufacturing
- Level: Expected
- Date: 3/27/2018
- Status: Complete, Available on Wiki
- Milestone name: Attempt 1st Needle Prototype
- Level: Expected
- Date: 4/11/2018
- Status: Complete
- Milestone name: Choice of Ultrasound System
- Level: Minimum
- Date: 4/14/2018
- Status: Complete
- Milestone name: Further Clinical Observations
- Level: Expected
- Date: 4/23/2018
- Status: Complete
- Milestone name: Documentation: Phantom Design and Manufacturing Plan
- Level: Expected
- Date: 4/23/2018
- Status: Complete, Available on Wiki
- Milestone name: 1st Geometric Phantom
- Level: Expected
- Date: 4/20/2018
- Status: Complete
- Milestone name: 2nd Geometric Phantom
- Level: Maximum
- Date: 4/27/2018
- Status: Complete
- Milestone name: Documentation: Phantom Test with Needle
- Level: Maximum
- Date: 5/4/2018
- Status: Complete, Available on Wiki
- Milestone name: Documentation: Phantom Test Data on Ultrasound System
- Level: Maximum
- Date: 5/7/2018
- Status: Complete, Available on Wiki
- Milestone name: Finalized Wiki Page with All Documentation
- Level: All
- Date: 5/10/2018
- Status: Complete
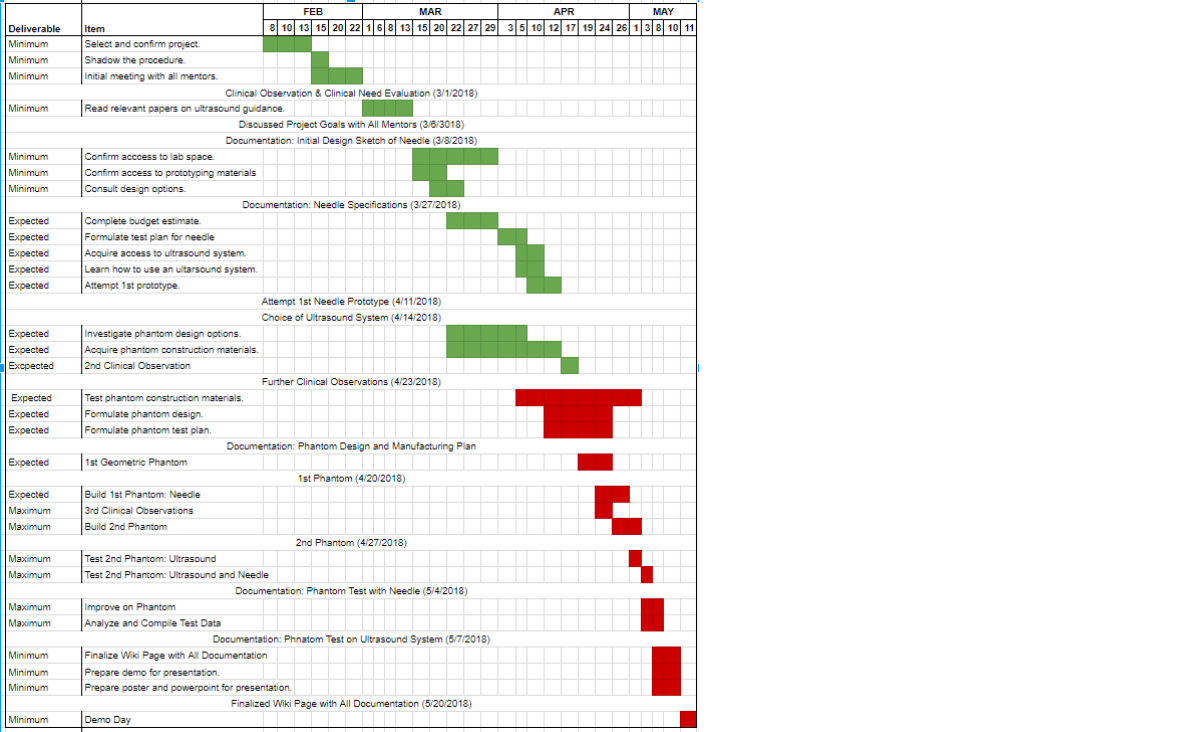
Here is a sample timeline. Note that in green is the work done prior to the Mid-Point presentation, while the work in red is the work done after the Mid-Point presentation up to the final presentation.
Reports and presentations
- Project Plan
- Project Background Reading
- See Bibliography below for links.
- Project Checkpoint
- Paper Seminar Presentation
- here provide links to all seminar presentations
- Documentation Collection
- Project Final Presentation
- Project Final Report
- links to any appendices or other material
Project Bibliography
Related recommended readings:
Bair, R. J., Bair, E., & Viswanathan, A. N. (2015). A radiopaque polymer hydrogel used as a fiducial marker in gynecologic-cancer patients receiving brachytherapy. Brachytherapy, 14(6), 876-880.
Banerjee, R., & Kamrava, M. (2014). Brachytherapy in the treatment of cervical cancer: a review. International journal of women's health, 6, 555.
Bell, M. A. L., Kuo, N. P., Song, D. Y., Kang, J. U., & Boctor, E. M. (2014). In vivo visualization of prostate brachytherapy seeds with photoacoustic imaging. Journal of biomedical optics, 19(12), 126011.
Damato, Antonio L., Megan Kassick, and Akila N. Viswanathan. “Rectum and bladder spacing in cervical cancer brachytherapy using a novel injectable hydrogel compound.” Brachytherapy 16.5 (2017): 949-955.
Guo, X., Kang, H. J., Etienne-Cummings, R., & Boctor, E. M. (2014). Active ultrasound pattern injection system (AUSPIS) for interventional tool guidance. PloS one, 9(10), e104262.
Nattagh, Khashayar, et al. “A training phantom for ultrasound-guided needle insertion and suturing.” Brachytherapy 13.4 (2014): 413-419.
Petric, P., & Kirisits, C. (2016). Potential role of TRAns Cervical Endosonography (TRACE) in brachytherapy of cervical cancer: proof of concept. Journal of contemporary brachytherapy, 8(3), 215.
Schmid, M. P., Pötter, R., Brader, P., Kratochwil, A., Goldner, G., Kirchheiner, K., … & Kirisits, C. (2013). Feasibility of transrectal ultrasonography for assessment of cervical cancer. Strahlentherapie und Onkologie, 189(2), 123-128.
Sharma, D. N., Rath, G. K., Thulkar, S., Kumar, S., Subramani, V., & Julka, P. K. (2010). Use of transrectal ultrasound for high dose rate interstitial brachytherapy for patients of carcinoma of uterine cervix. Journal of gynecologic oncology, 21(1), 12-17.
Viswanathan, A. N., Damato, A. L., & Nguyen, P. L. (2013). Novel use of a hydrogel spacer permits reirradiation in otherwise incurable recurrent gynecologic cancers. Journal of Clinical Oncology, 31(34), e446-e447.
Zhang, H. K., Lin, M., Kim, Y., Paredes, M., Kannan, K., Patel, N., … & Boctor, E. M. (2017, March). Toward dynamic lumbar punctures guidance based on single element synthetic tracked aperture ultrasound imaging. In Medical Imaging 2017: Image-Guided Procedures, Robotic Interventions, and Modeling (Vol. 10135, p. 101350J). International Society for Optics and Photonics.
Other Resources and Project Files
Here give list of other project files (e.g., source code) associated with the project. If these are online give a link to an appropriate external repository or to uploaded media files under this name space.2018-18