Contact Us
CiiS Lab
Johns Hopkins University
112 Hackerman Hall
3400 N. Charles Street
Baltimore, MD 21218
Directions
Lab Director
Russell Taylor
127 Hackerman Hall
rht@jhu.edu
Last updated: 5/10/18
The project aims at designing a tactile interface for detecting the breast lump and determining its location,shape,size and the type of lump.
According to the American cancer society, about 1,650,000 women will be diagnosed with breast lumps in the United States of America in 2018 and about 40,920 women will die from breast cancer. Some of the methods available for breast mass detection are MRI, Ultrasound, PET, Molecular based detection. But the golden method is the X-ray mammogram. Unfortunately, studies reveal that low-dose radiation increases breast cancer risk among these young high-risk women, and a careful approach is warranted. Hence the aim of this project is to develop a tactile imaging technique to detect breast lumps.
<fs larger>Minimum deliverable:</fs> [Expected Date of Completion: 4/2/18]
-Develop a tactile interface for breast lump detection
-Detect the presence, size, shape and location of lump
<fs larger>Expected deliverable:</fs>[Expected Date of Completion: 4/29/18]
-Detect the type(texture) of lump
-Develop a user interface for visualization of lump estimation
<fs larger>Maximum deliverable:</fs>[Expected Date of Completion: 5/10/18]
-Develop a phone application for data visualization
The deliverable was changed as we through it was more important to validate the developed process on different samples and determine the Success rate, False positive, False negative compared to developing an IoT.
-Evaluate Clinical diagnostic parameters
-Design interface probe
The proposed hardware structural diagram is as shown in the figure. The main components we will use are the tactile senor, a readout circuit, Matlab software for data processing and the GUI/ app for data visualization. The tactile sensor will be modeled in the shape of the breast (Eg, a brasier) to form a firm covering of the breast with a slight pressure. Research shows that a pressure of 70-90kPa is applied by a physician for breast palpation. Depending on the sensitivity of the sensor, the pressure will be optimized and a control system will be developed to enable detection of small lumps. The sensor will provide voltage readings inversely proportional to the firmness of the tissue. The voltage from the senor will be obtained through a readout circuit and the data will be processed in Matlab. The sensor will have to be calibrated to relate a voltage output to the firmness of the underlying tissue. The data will be signal processed followed by segmentation to obtain the size, shape and location of the lump and further be classified to obtain the texture of the mass. The visualization of the location, size, and shape will be provided in Matlab GUI and in an app that a physician can use to infer the results.
KEY COMPONENTS
HARDWARE
Tactile sensor: A low cost, pressure sensor made using conductive fabric (Fig. 2) was used for detection of lumps. The construction involves a piezoresistive fabric (NW-SLPA from Eeonyx) sandwiched between two layers of conductive fabric (Silver coated nylon, LessEMF). The conductive fabric forms a row and column matrix, where each intersection between a row and a column constitutes a sensing element. The arrangement is then held together using non-conductive fabric fusible interface.
The textile force sensitive resistors (FSRs) were characterized using an ADC (Arduino UNO). A normal force was applied directly to the sensing area of the textile cuff using a mechanical probe with a tip the same size as the sensing element. The operating range for the sensors is defined as the linear section of the sensor response curve on a log-log scale, which was found to be 0.5–20 N. This can be fit using a power trendline
Readout circuit: A standard multiplexed readout circuit was used (Fig. 4). As the resistance at each intersection decreases with increasing pressure, the pressure at each point is derived by measuring the potential difference across it. This is performed by activating each column m with a digital high signal (5V TTL) while deactivating other rows with a digital low. Each row n can then be read using a potential divider circuit, with each reading corresponding to the potential difference at point (m,n). The analog and digital pins are provided from the Arduino Uno board.
SOFTWARE ARCHITECTURE
The captured data had spatial resolution of 5 mm (total 36 spatial samples, 6 × 6 array), and the captured data was smoothed via a moving average over ten frames of sensing data. To increase the contrast of the imaged lumps and render them more robust to differences in lump depth or loading, enhanced images were formed by interpolating the raw measurements. Subsequently using this data, otsu thresholding and segmentation was performed to obtain the enhanced image
Lump Localization: Next step is to extract the connected components. 3-pixel neighborhood is used to find connected components. Two-pass Connected Component algorithm first labels all connected components with a unique label, and then unites some connected components together. The matrix output from the connected components search is a matrix array with lumps numbered from 1 to the total number of lumps present. From the output of the connect component search, the location of the lump, filled in area and the centroids are determined using the inbuilt Matlab function regionprops. The entire matrix region is divided into 5 regions based on the breast segments. The nipple area is determined to be the circle with center as 2D midpoint(xc,yc) of the array and a radius of 2 pixels. The left top, right top, left down and right down segments are extended to the horizontal and vertical midpoints of the matrix.
Visualisation: Due to the construction of the sensor, the output from all the conducting strips are not identical. Due to this, the voltage from the no lump region is low to be classified in bucket 3. In order to overcome this effect, a background subtraction algorithm was developed. An unloaded senor is read and interpolated which now form the base image I(x,y). This image is used as reference and is compared to the segmented image img (x,y). A MATLAB GUI was developed to display the image along with the area, centers, segments of location and the number of distinct lumps present along with their types.
 Fig 6. Plot before and After background subtraction
Fig 6. Plot before and After background subtraction
Lump type detection: The difference in stiffness gradient between the different types of lumps is used to classify the lumps. First, the depth of the lump is predicted from the output voltage using the SVM classifier. In order to determine the stiffness of the lumps, two assumptions are made. Firstly, the sensor is attached to a rigid surface compared to the breast tissues and the force exerted on the sensor will be translated into displacement of the tissues. Second, the displacement of the region with the lump as a reaction to the force is the same as the displacement of a normal tissue. Under the assumptions, the first principles (Hook’s law) is used to determine the stiffness of the lumps.
F = k. X
where k = stiffness of material F = Force output from sensor X = Displacement
SIMULATED SAMPLES
We fabricated simulated tissues with soft polymer (Ecoflex 00-30, Smoothon, Inc.; M100 modulus of 7 × 10 4 Pa). Samples included stiff lumps, in the form of solid balls (Young’s modulus 5 ×10 10 Pa) of varying radius and flat plates of varying dimensions and shapes (rectangle and plus) for the experiments on lump imaging. This material is stiffer than a biological tumor, but it provides an appropriate contrast in stiffness to the soft polymer and facilitates comparison with results from prior literature. Simulated tissues were fabricated with dimensions of 6 cm (width) × 6 cm (length) × 2.8 cm(height). We fabricated an array of tissues that varied in the depth h from the sample surface to the top of the lump and the diameter d of the solid. In this research, we selected different values of d in the experiment: d = 3,4,6,10,16,20 mm, which are in accordance with the range of observed breast tumor sizes. Five values of embedding depth h (the distance between the tissue surface and the top of the lump) were used: h =0, 2, 4, 6, 10 mm.
RESULTS
When present, the lump was a prominent central feature in tactile images of all the samples that possessed it. The sensitivity of the sensor without application of any force was suitable up to a depth of 6-8mm. To cover the entire screening depth of 12 mm, a force of 1.5N is required. The sensitivity of the sensor with an applied 1.5N force is within the usable range up to a depth of 10mm. In order to capture the depth information and maximize the sensitivity, the region is scanned three times with forces 0N, 0.8N and 1.5N applied on the sensing elements. For lump-containing tissues, as the lumps varied in depth, there was a decrease in voltage in the lump region of the image, and the decrease grew with increased embedding depth, yielding a qualitatively clearer image for lumps that were closer to the surface.
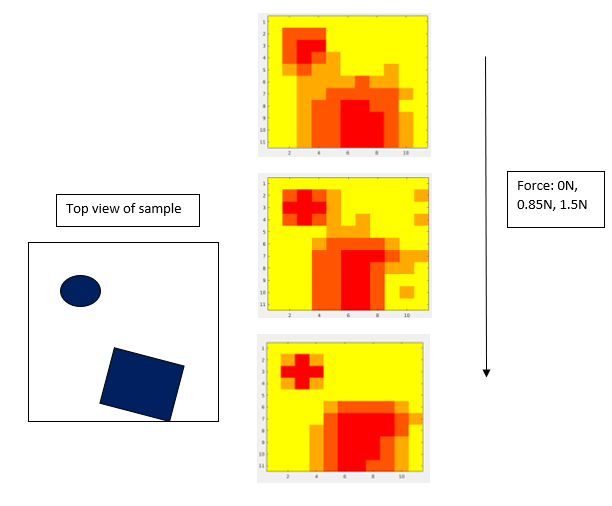 Fig 7. Plot at different applied force
Fig 7. Plot at different applied force
Experiments were conducted with phantoms by varying the depth and the size to determine the accuracy. The sensitivity of the developed method is 83% for lumps below 6mm depth and from 8 to 10 mm, the sensitivity is 45% for the lump size of 8mm. The experiments were repeated by varying the size of the lumps. For lumps larger than 4mm, the success rate is 67% up to a depth of 6 mm and for smaller lumps, the success rate of detection falls to 54% still a rate higher than the CBE in most countries.
Dependency Status
Training on fabrication of sensor Completed
Materials for sensor fabrication Completed
Readout circuit Completed
Phantom model of breast Completed
Calibration bridge Completed
Different texture samples Completed
Android phone/tab Not Started