Contact Us
CiiS Lab
Johns Hopkins University
112 Hackerman Hall
3400 N. Charles Street
Baltimore, MD 21218
Directions
Lab Director
Russell Taylor
127 Hackerman Hall
rht@jhu.edu
Last updated: 10 May 2015 19:33
Current brain imaging techniques are insufficient to fully understand the action of neurons in the brain. Existing techniques suffer a steep tradeoff between temporal and spatial resolution. In 2014, The Johns Hopkins University and the University of Copenhagen won an NIH planning grant for “Imaging In Vivo Neurotransmitter Modulation of Brain Network Activity in Realtime.”
Temporal resolution is the smallest time change at which an imaging modality can differentiate events. Action potentials (neural firings) usually take < 10 ms, so our imaging modality’s resolution must be in the order of milliseconds. PET, one of the best available brain imaging modalities, is far too slow, imaging activity in the order of minutes. fMRI images more in real time, but only shows regions of activity that are nowhere close to the neuron level. While the scans provided by PET and fMRI are useful, they don’t tell the whole picture of how the brain operates. Neurons fire in an asynchronous signal cascade. The signal begins at some neuron and propagates to different neurons as the event progresses. While these propagations often produce large amounts of activity in some region, the current regional imaging does not illustrate how the state of the response changes over time within the region. Improving the spatial and temporal resolution of brain imaging is essential to progressing our understanding of brain function, and ultimately to developing and implementing therapies.
Voltage sensitive dyes have been proven in vivo to respond to voltage changes at the microsecond time scale. Eriksson, Tompa, and Roland showed that voltage sensitive dyes in a ferret brain can distinguish between an ON neuron firings and the complementary OFF firings, in response to a visual stimulus of less than 100 ms. Measuring the fluorescence change of voltage sensitive dyes, the experimenters were able to observe the lag between different layers of neurons involved in the response.
The results in the ferret experimenter are exactly the kinds of data brain researchers need. However, the technique used in the experiment is totally unsuitable for human use. The procedure was a grueling 36 hour ordeal, complete with craniotomy. With the skull physically out of the way, introducing dyes to the brain and imaging the fluorescent response is relatively easy. Retain the skull, however, and the fluorescence of the dyes cannot be directly measured with a photodiode array.
Photoacoustic imaging allows the fluorescence change of the dyes to be measured through the skull, and through layers of tissue. Although the response of the dyes is the same, the photoacoustic system adds focused energy to magnify the response and transform it to mechanical waves that process better through the solid tissue.
The goal of the project is to develop noninvasive techniques for human brain imaging that provide good results in the millisecond time scale (the speed range in which neurons “fire”). The initiative aims to use intravenously delivered voltage sensitive dyes and/or pH sensitive dyes that can indicate the firing of individual neurons in the brain, and to record the activity as signaled by the dyes.
The initiative involves a multidisciplinary effort to solve problems that include developing safe and effective dyes and delivery of the dyes across the blood brain barrier. One challenge is recording the signal produced by the dyes once they are in the brain. The skull is an obvious barrier to a fluorescent signal produced in the brain. Additionally, observability of fluorescence below the outer layers of brain tissue is difficult. The hope is that photoacoustic imaging will allow noninvasive, fast, and accurate observance of signal changes, through the skull and layers of tissue. This imaging sub-goal is the object of our CIS project. To further this goal, we will performing experiments and analysis to characterize various dye candidates, to assist in optimizing the signaling process
Current Deliverables:
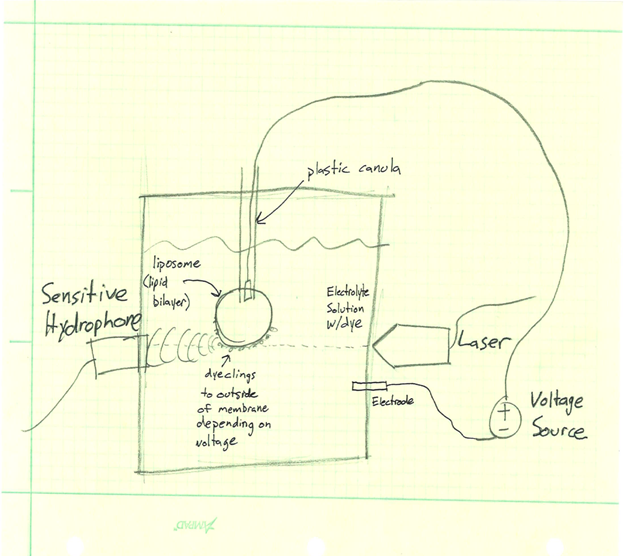
Photoacoustic imaging is broadly a two part system that involves an excitatory pulsed laser and a sensitive microphone to measure the acoustic output. The laser targets the desired region and pulses it with light of known wavelength. It is possible for the laser to shine through the skull and other tissue while remaining safe. The laser produces a thermomechanical response in the target. The target absorbs the energy from the light, causing thermal expansion and contraction. This movement produces pressure waves: an acoustic response. This pressure response can then propagate through tissue and even bone (for example from the brain through the skull) and can be recorded with an ultrasound pickup in contact with the exterior surface of the body.
The light energy of the laser is used to induce the acoustic response in the laser’s target. Because absorbance of light energy is an essential component of the process, the absorbance spectrum of the target is very strongly correlated to the output, an in fact different absorbance spectrums allow distinct constituents to be identified through the photoacoustic response. Since a change in fluorescence (such as that experienced by voltage sensitive dyes) is coupled with a change in absorbance, the potential for photoacoustic imaging being able to distinguish between changes in the dyes is very high.
In order to understand the photoacoustic response of the voltage sensitive dyes, we will be constructing test cells to characterize various dye’s response at different wavelengths, at different voltage potentials. For a given dye, we want to determine a map of the photoacoustic pressure response across a excitation wavelength range of 562 to 1064 nanometers, for voltages between -100 and 100 mV. Two sketches of potential testing systems can be found in the links below. One idea is to create a well inside of a polymer gel, line the well with dielectric material, and inject
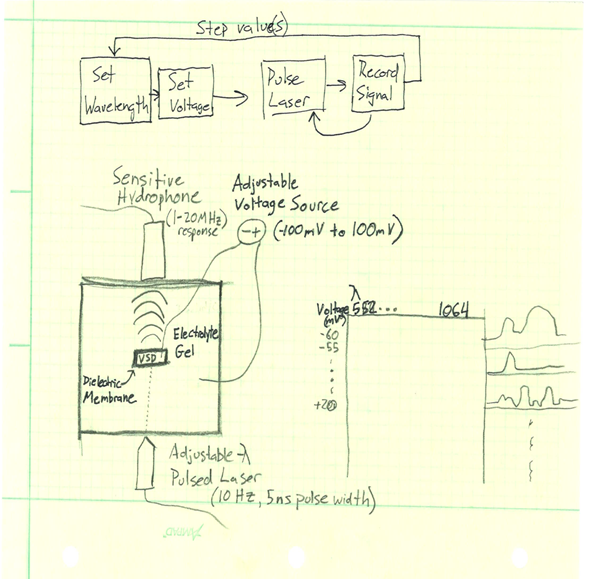
describe dependencies and effect on milestones and deliverables if not met
* Access to mentors
* Access to laboratory
* Laboratory training
* Access to dyes
* Synthesized liposomes from JHMI
Current Milestones:
Status update 3/20:
A gel mold has been constructed and tested. The mold produces a gel with 4 small (~100 microliter) size wells to contain candidate dyes.


Status update 4/2:
First wave of testing completed on baseline and on a mystery dye. Found ideal location of hydrophone. The wells hold ~ 3.5-4 uL of fluid.
Status update 4/7: Tried a new setup with light placed on top of gel and hydrophone on bottom. Due to problems with the optical cable wire, it was not possible to receive any data. The gel phantom has been redesigned to contain wells on the four corners.
Status update 4/9: Testing resumed on new gel phantom design. 2 dyes were tested and data analysis will be done soon.
Status update 4/20: Acquired spectrum for various dyes while under baseline conditions. We are hoping to get started on characterizations under different pH values soon
Status update 4/29: Were able to test 3 dyes under various pH conditions. Ideally we wanted more but we are going to move on to skull phantom testing soon
Status update 5/2: Tested a single dye with skull phantom. If we have more time we will test more
Here give list of other project files (e.g., source code) associated with the project. If these are online give a link to an appropriate external repository or to uploaded media files under this name space.