Contact Us
CiiS Lab
Johns Hopkins University
112 Hackerman Hall
3400 N. Charles Street
Baltimore, MD 21218
Directions
Lab Director
Russell Taylor
127 Hackerman Hall
rht@jhu.edu
Last updated: May 5, 2015 at 22:00
Microvascular surgeries require a great degree of precision, accuracy, and stability for successful operations. In many cases, the microvascular anastomosis of vessels during free tissue transfer are the most technically challenging and critical portion of these already long procedures. Controlling hand tremor during vein suturing is invaluable, and even skilled surgeons who have spent years perfecting a steady hand still exhibit a slight tremor. The negative effects of hand tremors are magnified at the microsurgical scale. Existing systems such as the daVinci Surgical System have been hastily adapted to assist in microsurgery; however, no robotic system has been developed to specialize in cooperatively controlled robotic microsurgery. The Robotic Ear Nose and Throat Microsurgery System (REMS) was developed by Dr. Taylor, Kevin Olds, and Marcin Balicki to address this pressing issue. The robot was initially built and tested for laryngeal phonosurgeries, however its application will be expanded to include microvascular anastomosis. This project will attempt to redesign the tool set and attachment mechanism of the REMS for seamless integration of suture needle holders, improving the ease, efficiency, and accuracy of microsurgical procedures.
Microvascular surgery is at the cornerstone of several reconstructive procedures throughout Otolaryngology – Head and Neck Surgery and has become commonplace in training programs across the country, with more than one in eight academic Otolaryngologists reporting microvascular training. Specifically, free flaps remain the preferred method of reconstruction for complex defects after ablative procedures including oncologic resections. These procedures have continued to improve over the past 10 years and currently demonstrate success rates exceeding 95% in the literature. However, these procedures continue to have a high overall cost due in large part to lengthy hospital stays and long operating times. In many cases the microvascular anastamosis of vessels during free tissue transfer remains the most technically challenging and critical portion of these long procedures. In addition to technical complexities of microvascular techniques, a surgeon’s inherent dexterity and essential tremor are limiting factors to operative time and surgical efficiency. Novice surgeons struggle with mastery of these techniques. This is especially true in microvascular surgery where the hard skills and inherent tremor of the operator are magnified. To our knowledge no other robotic system exists to enhance the surgeon’s ability to perform microvascular surgery.
The Robotic Ear Nose and Throat Microsurgery System (REMS) is an external, non-invasive gantry unit that was developed as a stabilizing mechanism for the surgeon’s primary instrument. A force-feedback control system eliminates the surgeon’s intrinsic tremor, enhances control over fine motor movements and maintains one-to-one haptic feedback for the user. The applications for such a device extend well beyond microvascular surgery. Within the domain of Otolaryngology, these include, but are not limited to otology, laryngology, sinus and skull base surgeries. We believe that the validation of this system for anastomosis and its initial proof of concept through a user study will be the first steps towards eventual incorporation in various operative aspects of microvascular surgery.
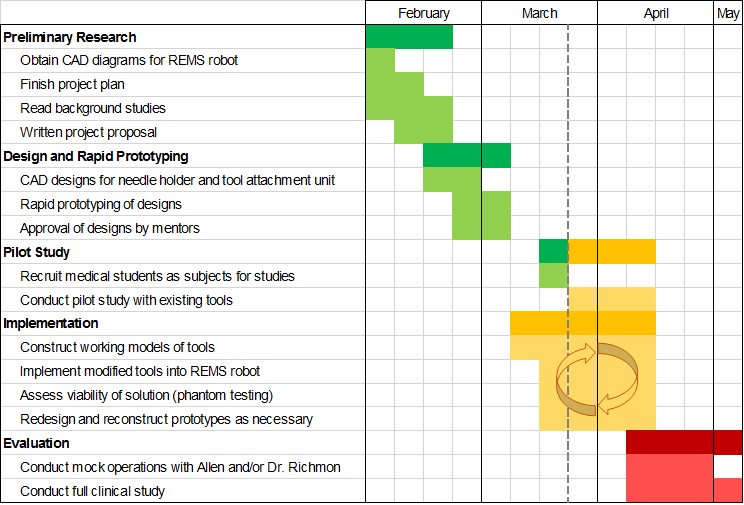
The design process used for the development of the surgical instruments will follow a standard engineering design sequence, beginning with brainstorming. Once optimal designs have been identified based on desired specifications, computer aided models will be created to aid in the prototyping process. Through the use of machine shop tools and materials, the instruments will be fabricated and implemented to be used with the REMS, including applying changes to the movement algorithm to account for the different tools. The testing phase will consist of the use of phantoms to evaluate the effectiveness of the new instruments. Based on feedback from the results of the phantom tests and from mentors, the instruments will be refined or redesigned to meet the specifications.
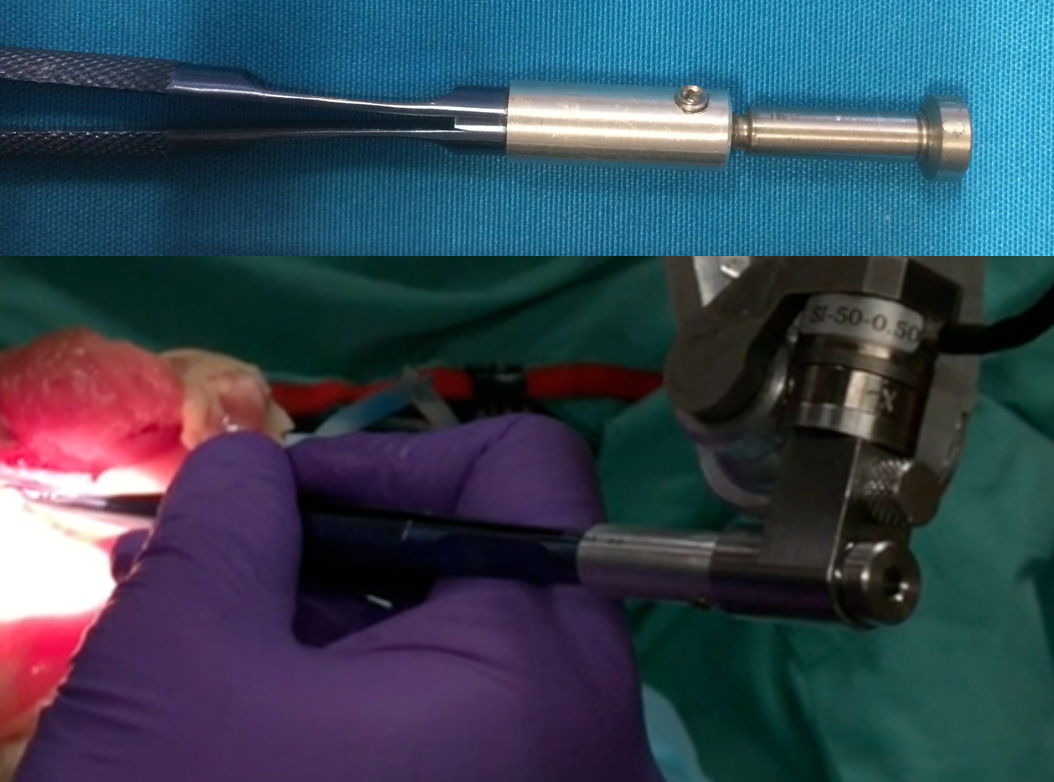
The first constructed piece was an adapter to rigidly attach a set of pre-fabricated forceps to the REMS, shown to the left. This tool will be used to perform the first round of the user study, both manually and using the REMS.
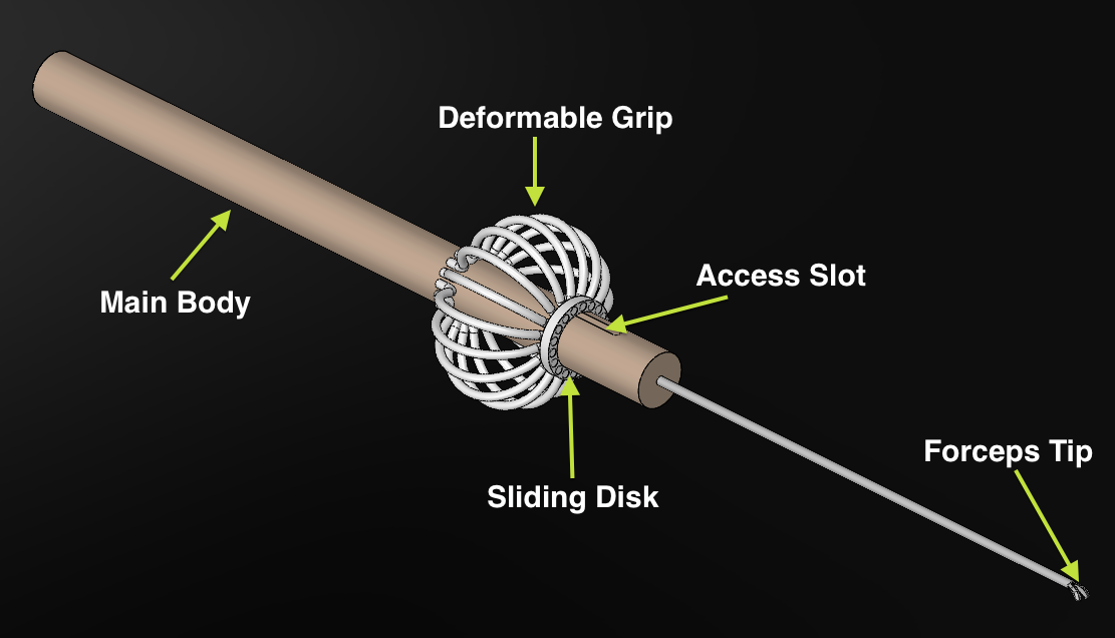
The figure to the left shows a model of the first proposed design. Following the Eye Robot's example of a deformable grip, this tool would provide improve ergonomics using a symmetrical design. The tool will be constructed using stainless aluminum for the main shaft and pre-made tips, removed from professionally made forceps. The deformable grip will be constructed using stainless spring steel to allow for actuation in response to applied force.
It was found that this design was too difficult to construct due to the physical limits of the spring steel wires. For such short lengths of wire, the wires were too rigid to properly bend to actuate the tips.
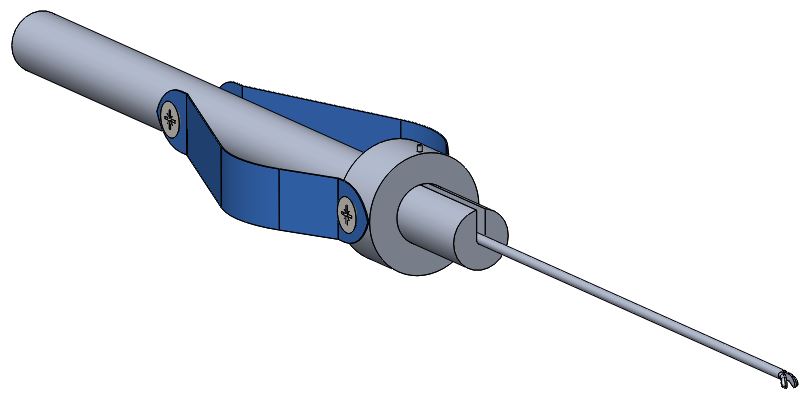
The figure to the left shows the updated model of the custom tool. Using sheets of spring steel, it was possible to create a deformable grip that can actuate the tips. It can be noted that this design does not use any permanent binding techniques, such as welding. Rather, all parts are removable, making it ideal for troubleshooting problems and improving the modularity of the system.
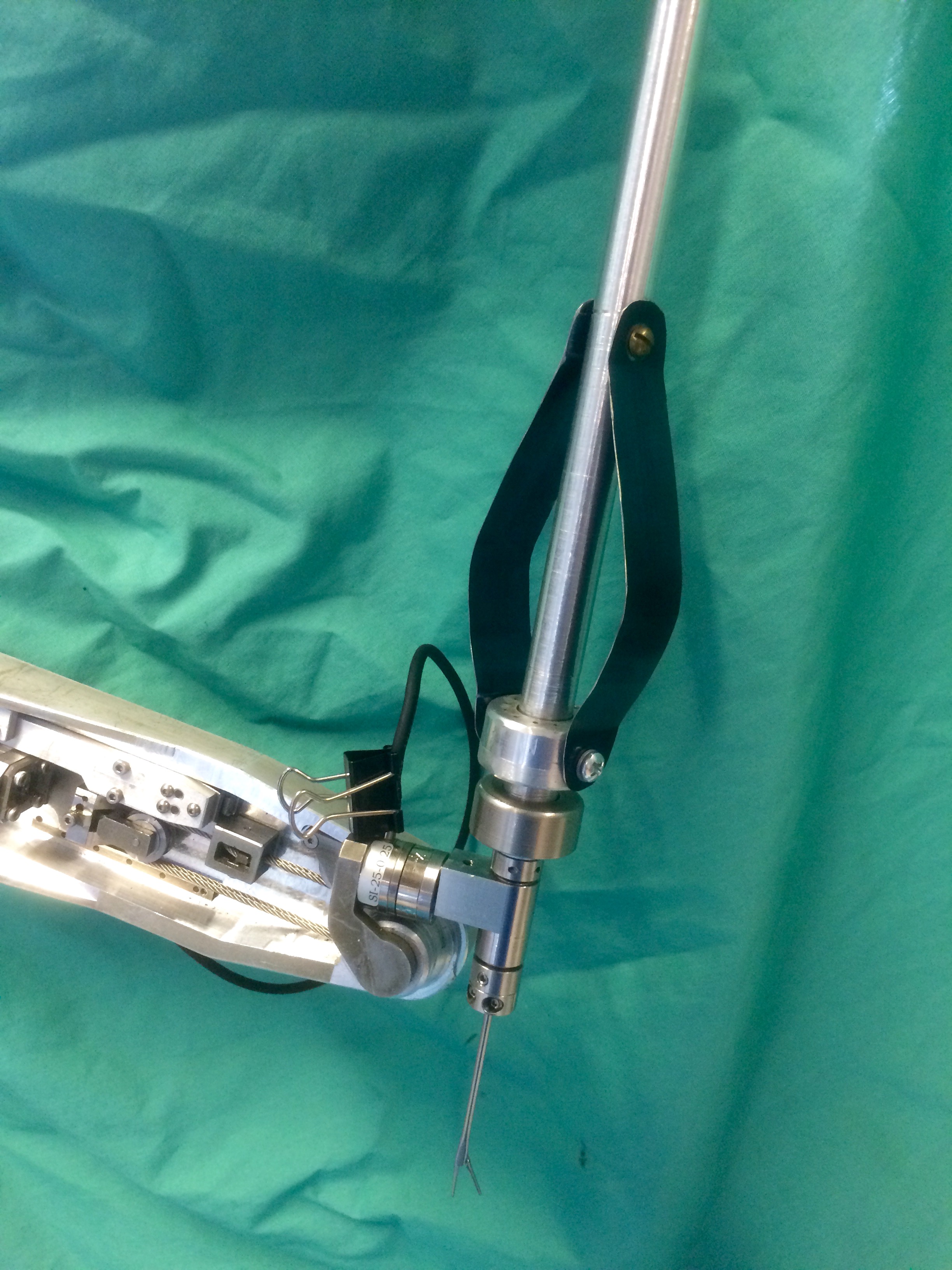
The prototype was then implemented with the REMS, attaching rigidly to the force sensor in a manner similar to the laryngeal tools. By operating the tool above the force sensor, the robot arm can remain closer to the surgical area, preventing the need for large movements by the arm in response to a user input.
The user study will be performed using a sample of at least 10 medical students, with an additional set of users being made up of 5 medical residents and possibly 1 other attending physician. The experiment is set up as shown below, using store-bought chicken as a source of the vasculature.

Following preliminary design testing, mock operations will be performed by Dr. Richmon and Allen Feng to determine the effectiveness of the new tools in a microvascular anastomosis procedure.
Finally, extensive studies will be performed using sufficient samples of medical students to perform similar procedures, first, a pilot study using existing tools, and secondly, an evaluation of the newly designed tools. Major factors of evaluation include ease of use, ergonomics, efficiency, and accuracy of movement.
STATUS: All dependencies have been resolved
Machine shop certification
Access to REMS
Materials to design prototypes
Scheduling of mock operations and study
Recruitment of medical students for studies
Key:
Spiegel JH, Polat JK. Microvascular free flap reconstruction by otolaryngologists: prevalence, postoperative care, and monitoring techniques. Laryngoscope 2007; 117: 485–490.
Chien W, Varvares MA, Hadlock T, et al. Effects of aspirin and low-dose heparin in head and neck reconstruction using microvascular free flaps. Laryngoscope 2005; 115: 973–976.
Top H, Sarikaya A, Aygit AC, et al. Review of monitoring free muscle flap transfers in reconstructive surgery: role of 99m Tc sestamibi scintigraphy. Nuclear Med Commun 2006;27: 91–98.
Rosenthal E, Carroll W, Dobbs M, et al. Simplifying head and neck mirovascular reconstruction. Head Neck 2004;26: 930 –936.
Suh JD, Sercarz JA, Abemayor E, et al. Analysis of outcome and complications in 400 cases of microvascular head and neck reconstruction. Arch Otolaryngol Head Neck Surg 2004;130:962–966.
Urken ML, Buchbinder D, Costantino PD, et al. oromandibu- lar reconstruction using microvascular composite flaps: re- port of 210 cases. Arch Otolaryngol Head Neck Surg 1998; 124:46 –55.
Ryan MW, Hochman M. Length of stay after free flap recon- struction of the head and neck. Laryngoscope 2000;110: 210 –216.
Olds K, Chalasani P, Pacheco-Lopez P, Iordachita I, Akst L, Taylor R. “Preliminary Evaluation of a New Microsurgical Robotic System for Head and Neck Surgery”, IEEE/RSJ International Conference on Intelligent Robots and Systems, 2014.
Nimmons, G., Chang, K., Funk, G., Shonka, D., & Pagedar, N. (2012). Validation of a Task-Specific Scoring System for a Microvascular Surgery Simulation Model. The Laryngoscope,122(10), 2164-2168.